FDA and NIH investigate HT-DLS for nanoparticle stability

One of the major challenges in the development of nanoparticles (NPs) for drug delivery, phototherapy and diagnostics is assessing their stability under physiological conditions and, for photothermal therapies, under therapeutic conditions. The pH and ionic strength of standard blood and the intracellular matrix can negate the effects of charge-charge repulsion in such particles, lead to aggregation and even flocculation, hence preventing the penetration of the NPs into the targeted cells.
Another issue of concern is interaction of NPs with soluble blood components such as serum albumin and IgG. The attachment of these proteins to the NPs can be detrimental in terms of increased aggregation, but also via biochemical impact on delivery and/or reduction in efficacy of the NP payload. At the elevated temperatures typical of photothermal therapy, other types of degradation may occur such as dissociation of a polymer micelle.
In a therapeutic NP development effort, stability under physiological conditions and multiple temperature regimes must be evaluated for multiple candidate NPs, over an extended time scale. In our Featured Publication 36, Ashwinkumar Bhirde et al. of the FDA and NIH successfully demonstrate the use of Dynamic Light Scattering using the DynaPro DLS Plate Reader II to assess the colloidal stability of a several model NPs, their interaction with serum albumin, and the impact of the interaction on colloidal stability. The study greatly benefited from the full automation and high throughput provided by the DynaPro PR2. According to the authors, "this... high-throughput method can accomplish sophisticated hydrodynamic size measurement protocols within days instead of years it would take conventional hydrodynamic size measurement techniques to achieve a similar task."
The study included measurements over a period of 3-5 days and temperature cycles between 37°C and 60°C, in both water and a medium containing bovine serum albumin (BSA). Au NP sizes ranged from 10-100 nm, and larger when PEGylated. One of the key results was the robustness of PEGylated Au NPs against the attachment of BSA, proving that high-throughput, plate-based DLS can readily distinguish between NPs coated and uncoated with protein as well as aggregation.

Authors from the FDA and NIH have published on using the DynaPro Plate Reader II to study stability of a model colloidal system under physiological conditions

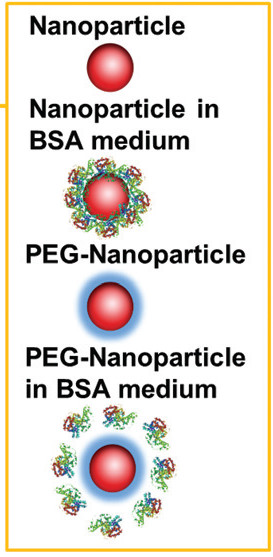
Illustration from Figure 1 of Bhirde et al 2017
