How can Wyatt Technology streamline ADC characterization with SEC-MALS

Introduction
Wyatt Technology offers a comprehensive Antibody-Drug Conjugate (ADC) Guidance Manual and a dedicated ADC analysis module to help laboratories accelerate workflows and achieve consistent results across runs and multiple sites.
The ADC characterization process utilizes multi-angle light scattering (MALS) with the DAWN™ MALS instrument, combined with UV absorbance at multiple wavelengths and the Optilab™ differential refractive index (dRI) detector, coupled to size exclusion chromatography (SEC). This robust approach enables detailed analysis of key ADC attributes, including drug-antibody-ratio (DAR), molar mass, and aggregation.
In this article, we highlight the tools and resources available to support your ADC characterization needs.

A SEC-MALS system comprises HPLC system with Wyatt’s MALS and dRI instruments.
ADC SOP Guidance Manual
The ADC Guidance Manual serves as a go-to resource for data acquisition and analysis by SEC-MALS for ADCs and related analytes, like antibody-oligonucleotide conjugates (AOC).
We describe method settings, data acquisition, and analysis in detail to support development of a Standard Operating Procedure. Key topics covered include system considerations, column selection, recommended LC parameters, data acquisition, and analysis, along with expert guidance on data interpretation, analysis, and troubleshooting.
Organized for clarity and ease of use, the manual is fully searchable and allows users to print specific sections as needed.

ADC Guidance Manual.
Dedicated ADC analysis module
The dedicated ADC analysis module within Wyatt's ASTRA software simplifies ADC characterization by enabling critical attribute analysis in a single run. This analysis module goes beyond traditional protein-conjugate analysis by enabling collection and analysis with two UV wavelengths simultaneously for increased sensitivity and precision.
This module quantifies essential parameters, including total conjugate molar mass, composition, drug-antibody-ratio (DAR), and aggregation. Additionally, the module supports characterization of antibodies with larger payloads, such as antibody-oligonucleotide conjugates (AOCs).

EASI Graph of the drug-antibody ratio versus elution time or volume.
DAR analysis
SEC-MALS-UV-dRI provides a simple and accurate method for determining the drug-antibody-ratio (DAR) of ADCs during product development and quality control. The ASTRA software integrates UV/Vis and dRI signals to separate the contributions of the protein and drug conjugate, enabling calculation of molar mass distributions of each component. These distributions can be averaged across the entire sample or analyzed as a function of elution volume.

EASI Table displaying Antibody-Drug Conjugate Analysis results peak-by-peak.
Extinction coefficient determination
The extinction coefficient of ADCs is altered by conjugation with linkers and drug payloads, which complicates UV/Vis concentration analysis. The SEC-MALS-UV-dRI method addresses this challenge by empirically measuring the extinction coefficient, ensuring consistent and accurate UV/Vis quantification.
References on protein extinction coefficient measurement by SEC-MALS:
Wen, J., et al. Techniques in protein chemistry, vol. 8, pp. 113-119. Academic Press, 1997.
Miranda-Hernández, M., et al. Analytical and bioanalytical chemistry 408, no. 5 (2016): 1523-1530.
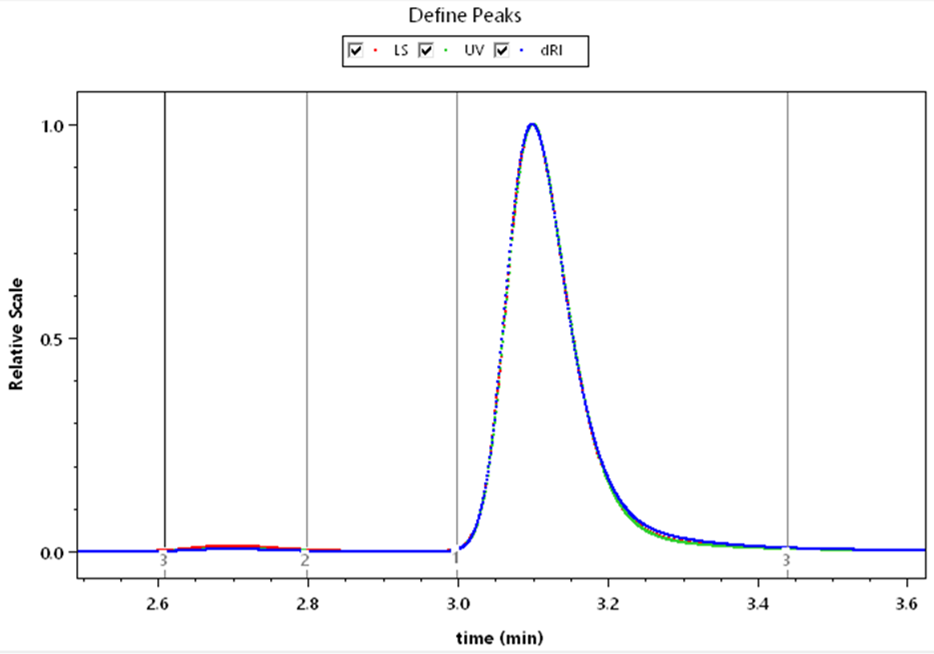
UV extinction coefficient determination from RI.
Conclusion
Wyatt provides a full suite of instruments and methods for complete quantitation and characterization of your proteins and ADC products, including SEC-MALS, and DLS solutions. To learn more about our 21 CFR Part 11 compliant solutions for ADC analysis, please visit the compliance section of our website.
Interested in learning more?
Contact our experts here in Customer Support.
We’re happy to help! Call +1 (805) 681-9009 option 4.
