Solutions for Lipid Nanoparticle Charaterization in Product & Process Development
Lipid nanoparticle (LNP)-based delivery of pharmaceuticals holds much promise for novel gene therapies, oncology, and effective, rapid-response vaccines. Platform technologies that characterize and quantify LNP attributes across R&D, production and quality control will help realize that promise. Click here to read a SelectScience editorial article on the latest advancements and expert opinions on characterizing LNPs for drug delivery.
Wyatt’s suite of solutions for analyzing critical LNP quality attributes including:
- Average size, polydispersity and detailed size distributions
- Particle concentration
- Size-based nucleic acid (NA) payload distribution
- Stability under a variety of stressors
Many of these assays can be streamlined and automated for high throughput.
Light scattering techniques are simple to operate and enhance productivity, while directly accessing the core biophysical properties that define these CQAs. Continue reading to learn about Wyatt’s solutions to industry and regulatory needs for LNPs and other nanopharmaceuticals.
Wyatt’s products characterize both viral and non-viral gene vectors. Publication list PL9008: Key publications on the characterization of gene vectors by light scattering lists some peer-reviewed publications describing how.
Listen to the webinar “Size, Concentration, Payload and Quality: Comprehensive Characterization of LNP-RNA Therapeutics by Light Scattering”.
Particle size distributions
Particle size, which affects biodistribution and cellular uptake, is one of the most important properties of LNPs. Multi-angle and dynamic light scattering (MALS, DLS) are utilized at different stages of LNP development, production and quality control.
Formulation development & quality control
Rapid screening of formulation conditions to produce LNPs in the desired size range and concentration reduces manual effort, improves product consistency and speeds up time to market. High-throughput DLS using a DynaPro™ Plate Reader (DPR) measures size and particle concentration in standard 96, 384 or 1536 well plates, providing maximum convenience and lowest consumables cost without sacrificing accuracy or sensitivity.
High-resolution size distributions with FFF-MALS
Field-flow fractionation coupled to multi-angle light scattering (FFF-MALS) is the premier method for accurate and quantitative LNP size distributions, providing levels of detail, accuracy, repeatability and automation not possible with other methods. The implications of FFF-MALS for regulatory aspects of LNP development were presented by Dr. Fanny Caputo in the webinar Measuring physical properties of liposomes and LNPs for RNA delivery with multidetector asymmetric-flow field-flow fractionation.
ASTRA’s Number Density method provides direct measurement of particle size and concentration derived from the light scattering intensity. These calculations can be applied to a variety of common gene delivery vehicles, including viruses, virus-like particles and lipid nanoparticles. Learn more about the sensitivity and linearity of this method in this paper published by scientists at the CDC: Quantitation of influenza virus using field-flow fractionation and multi-angle light scattering for quantifying influenza A particles.
Wyatt’s solutions for characterizing a variety of gene therapy products are described in the brochure Characterization of Biologics and Complex Drugs.
More than just size distributions may be determined with FFF-MALS. A variety of applications of FFF-MALS to characterization of liposomes and LNPs, including preclinical characterization of Lipidots and the kinetics of drug transfer between nanocarriers and a proxy for the cellular membrane, are presented in the webinar Characterization of Lipid Nanoparticle Drug Formulations and in WP2608: Lipid nanoparticle and liposome characterization with FFF-MALS.
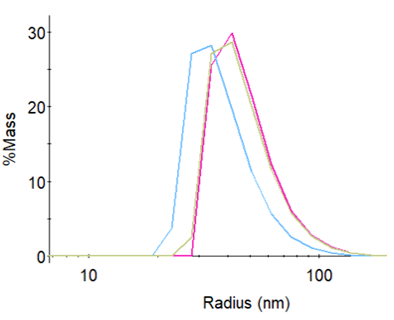
Size distribution of three LNP preparations measured by a DLS Plate Reader.
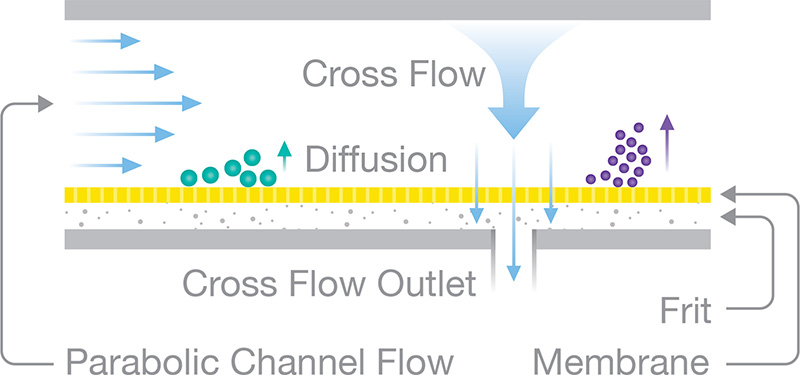
Field-flow fractionation (FFF) separates particles according to diffusion coefficient, i.e. hydrodynamic radius.
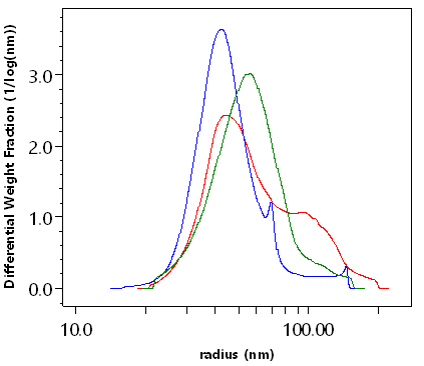
Size distribution of the same three LNP preparations as above but measured by FFF-MALS.
Payload quantification
Online payload analysis by MALS-UV-dRI following SEC or FFF
ASTRA's LNP Analysis module quantitates the nucleic acid weight fraction using online MALS, UV260 and dRI detectors coupled to SEC or FFF. The same data simultaneously quantify many other quality attributes, such as size distribution, particle concentration, morphology, encapsulation efficiency, mRNA concentration and lipid concentration. The method was validated against conventional offline measurements in “Enabling online determination of the size-dependent RNA content of lipid nanoparticle-based RNA formulations.”
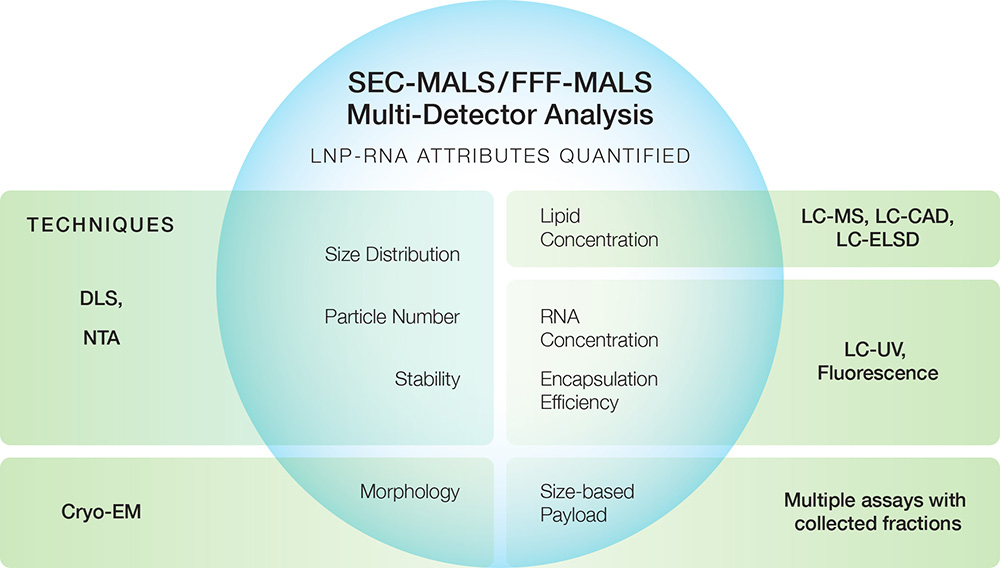
The FFF-MALS-UV-dRI system
Though SEC has been used to separate LNPs, FFF is the tool-of-choice for LNP and other bioNPs owing to its tunable resolution and better mass recovery. Wyatt’s FFF-MALS system for LNP payload analysis comprises an Eclipse™ FFF instrument, DAWN™ MALS instrument (with optional embedded WyattQELS™ DLS module), Optilab™ differential refractive index (dRI) detector and industry-standard HPLC pump, autosampler and UV detector from Agilent Technologies. A fraction collector may be added downstream.
The entire system is orchestrated by VISION™, Wyatt’s innovative software for FFF method design and instrument control. Data are collected and analyzed with ASTRA™, the premier software for MALS and DLS. VISION and ASTRA are both available in 21 CFR Part 11 compliant versions.
Wyatt’s product lines and application range are outlined in Solutions for Macromolecular and Nanoparticle Characterization.

Wyatt’s FFF-MALS system comprising Eclipse, DAWN and Optilab.
mRNA/DNA
mRNA biophysical attributes
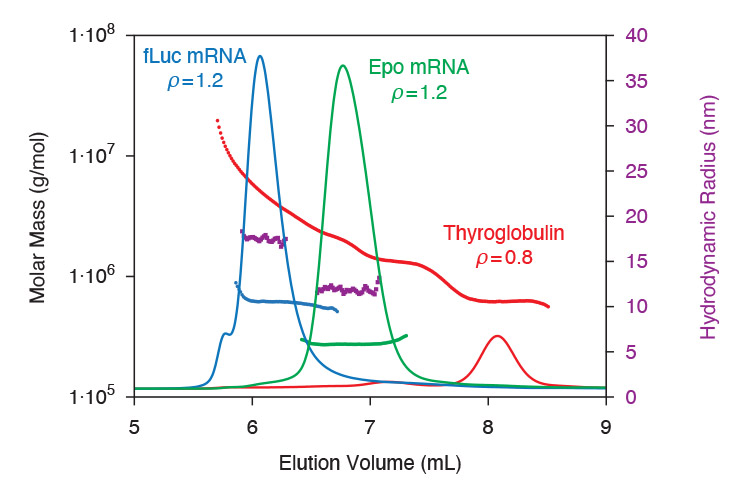
As described in application note AN1616: SEC-MALS methods for characterizing mRNA, key biophysical attributes of mRNA are quantified by SEC-MALS plus DLS. This analysis determines molecular weight, percentage of aggregates, radius of gyration Rg and hydrodynamic radius Rh. The relationship between Mw and Rg identifies single- or double-stranded RNA, while the ratio Rg:Rh indicates molecular conformation. This method can be readily adopted to further study how these attributes are affected by mRNA preparation, formulation and storage conditions.
Process analytical technology for LNPs
LNP process development and analytics
Once a successful LNP/mRNA platform has been developed, scaling up the process is facilitated by real-time multi-angle light scattering (RT-MALS). With ultraDAWN™ either in-line or on-line, continuous monitoring of critical quality attributes like particle size and concentration is achieved. Real-time monitoring of liposome size in a production environment is described in AN8006: In line monitoring of liposome size by RT-MALS.
Real-time MALS for PAT
RT-MALS can be applied during process development, to eliminate time-consuming off-line measurements of fractions by DLS or particle tracking analysis (PTA), as well as to monitor and flag potential deviations during full GMP production. In GMP operation, OBSERVER™ RT-MALS software becomes an OPC-UA server in order to integrate with process control. To learn more, view the webinar Real-Time Process Analytics and Control for Vaccine Nanoparticles and Macromolecules or request the ultraDAWN brochure.




