Measuring Size with Light Scattering
The size of macromolecules or nanoparticles is measured via two light scattering methods: Multi-Angle static Light Scattering (MALS) and Dynamic Light Scattering (DLS). Each technique contributes critical pieces of the characterization puzzle.
Introduction
Both Multi-Angle static Light Scattering (MALS) and Dynamic Light Scattering (DLS) provide non-perturbative, solution-based size measurements based on absolute analyses, independently of calibration standards. These techniques are complementary and orthogonal, analyzing different properties of the scattered light.
Size by MALS
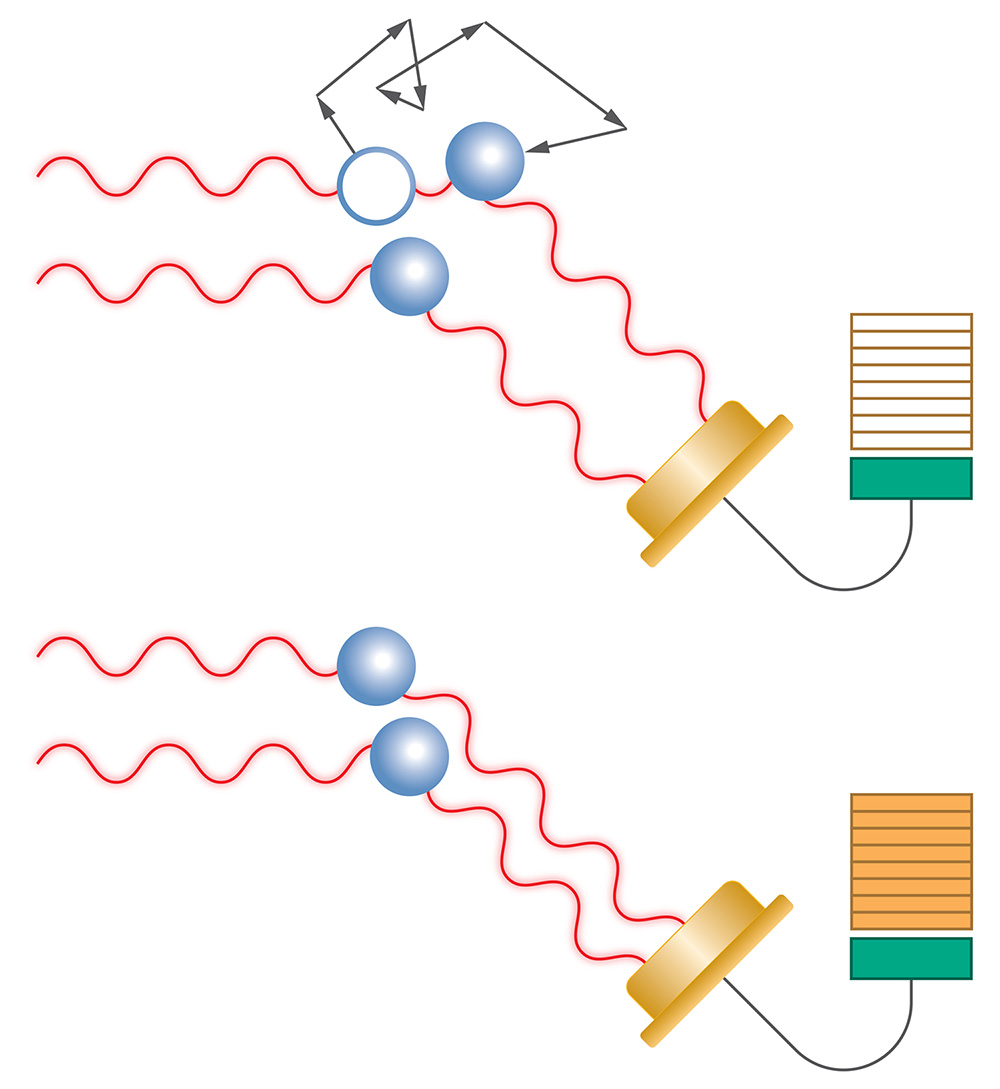
MALS examines the angular dependence of the time-averaged scattering intensity to determine the mass-averaged root mean square radius Rg (a.k.a. 'radius of gyration') from 10 nm to several hundred nanometers, independent of shape, as described in Classical Light Scattering Theory.
If the sample conforms to specific shape models such as sphere, rod, random coil or coated sphere then the radius or rod half-length can be determined to as much as 1000 nm by a DAWN™ 18-angle MALS detector. miniDAWN™ and microDAWN™ 3-angle detectors characterize sizes up to 50 nm, independent of shape, and up to 150 nm if the sample conforms to shape-specific models.

MALS determines size via the angular dependence of the scattered intensity.
Size distributions by on-line (fractionated) MALS
Coupling a MALS detector to a fractionation method such as size exclusion chromatography (SEC-MALS) or field-flow fractionation (FFF-MALS) is the most common method for MALS characterization of size distributions of homogeneous or heterogeneous samples. MALS analysis provides the rms radius Rg of the analyte at each elution volume, independently of retention time. Since the detection volume of the MALS flow cell is much smaller than a typical monomeric peak eluting from SEC or FFF, MALS provides accurate size distributions limited in resolution only by the separation technique.
Applications
SEC-MALS and FFF-MALS determine the size distributions of:
- Biopolymers such as polysaccharides
- PEGylated proteins and large protein complexes such as virus-like particles
- Synthetic polymers
- Liposomes or exosomes
- Large protein aggregates
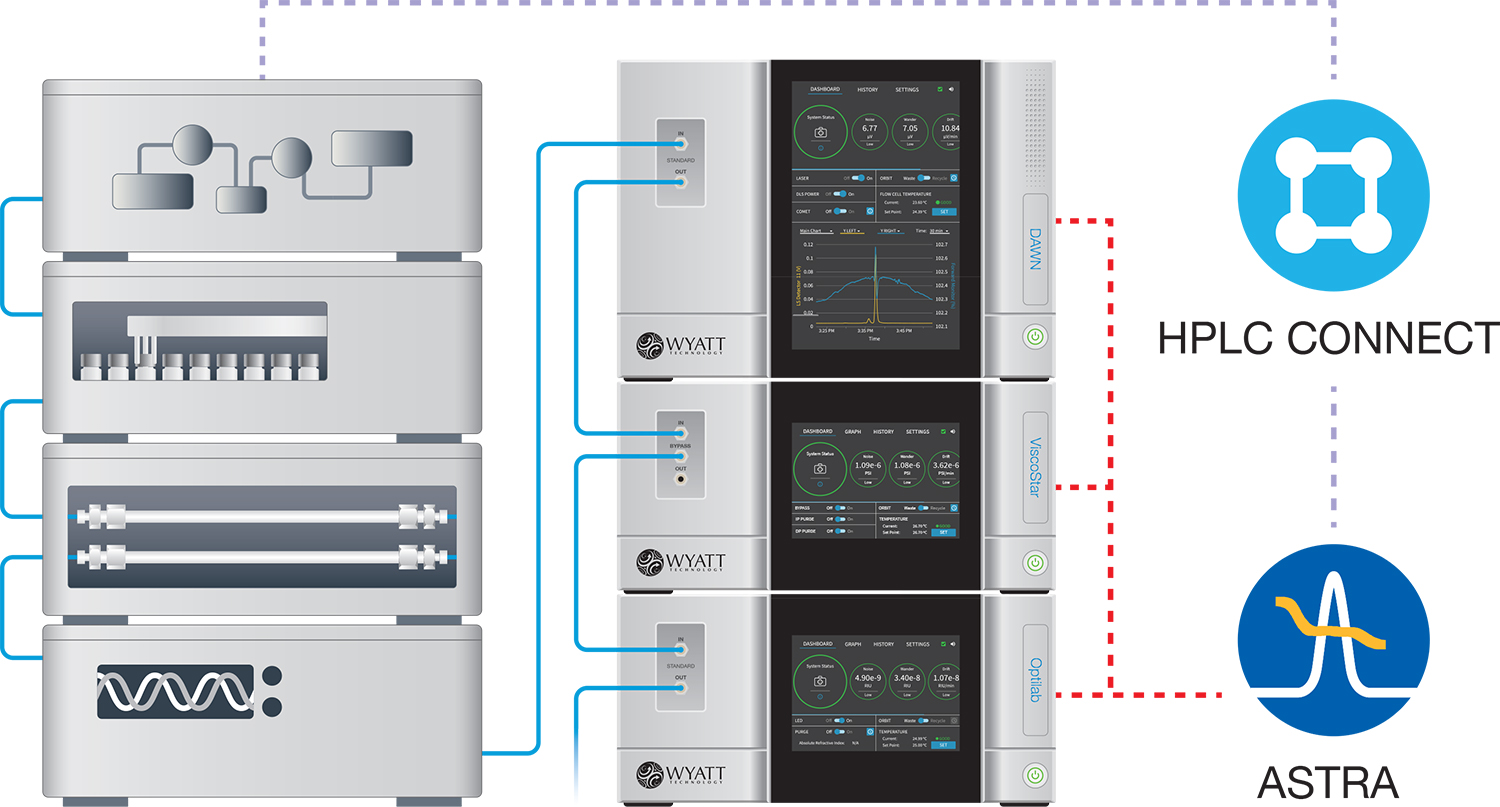
Average size by batch (unfractionated) MALS
An unfractionated, or 'batch' measurement, provides the Z-average radius Rg,z of the entire analyte content in solution. Batch MALS for size measurements is carried out by injecting the sample directly into the DAWN or miniDAWN flow cell. Historically, batch measurements have been laborious and prone to operator error; the Calypso™ automates batch MALS for accurate, reliable and rapid results.
Applications
Batch MALS is often used to analyze:
- Very large, unstructured polymers that are not amenable to fractionation
- Large protein assemblies that dissociate under the dilution and shear inherent to fractionation
- Polymers that form gels or viscous solutions that may impede flow paths

MALS for Process Analytics
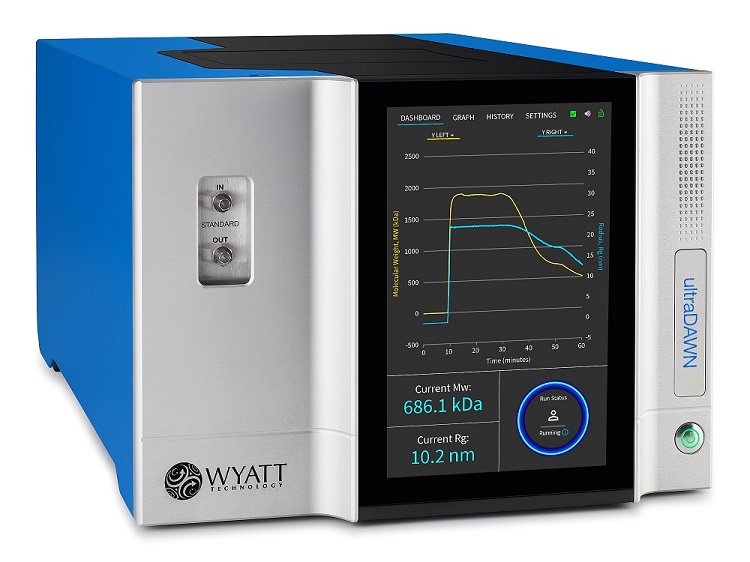
In addition to standard analytical applications of MALS, it may be used to develop, monitor and control manufacturing processes, directly in the pilot plant or on the manufacturing floor. The ultraDAWN™ along with OBSERVER™ software determine and report the z-average rms radius (Rg) of protein or polymer solutions as well as z-average radius (Rz) of nanoparticles passing through the instrument in real time; this is known as RT-MALS.
Online or batch MALS
DAWN - The most sensitive MALS detector available, anywhere. Incorporates detectors at 18 angles to determine molar masses from 200 Da to 1 GDa and radii from 10 – 500 nm.
- Standard option: ambient temperature
- Heated/cooled option: -15 °C to +150 °C
- High-temperature option: ambient to +210 °C
The DAWN offers special options to handle fluorescent samples: fluorescence-blocking filters and an infrared, 785 nm laser.
miniDAWN - Second only to the DAWN in sensitivity. Incorporates detectors at 3 angles to determine molar masses from 200 Da to 10 MDa and radii from 10 – 50 nm. Ambient only.
microDAWN - The first MALS detector for UHPLC, with interdetector dispersion as low as 1.5 µL. Incorporates detectors at 3 angles to determine molar masses from 200 Da to 20 MDa and radii from 10 – 50 nm. Ambient only.
Calypso - Preparative and sample delivery system and software for carrying out and analyzing automated concentration or composition gradients in a MALS detector. Automates reliable and repeatable Zimm plots as well as measure time- or composition-dependent size in a DAWN or miniDAWN.
Software
ASTRA™ - Our comprehensive software solution for MALS and DLS analysis in chromatography, AF4 or batch mode. ASTRA is available in a 21CFR(11) compliant version and offers additional options such as particle analysis.
CALYPSO™ - The CALYPSO software does not analyze DLS data, but can be set to control dilutions or composition gradients delivered to a MALS or Mobius detector and trigger DLS data acquisition by Wyatt's ASTRA or DYNAMICS™ software packages.

Size by DLS
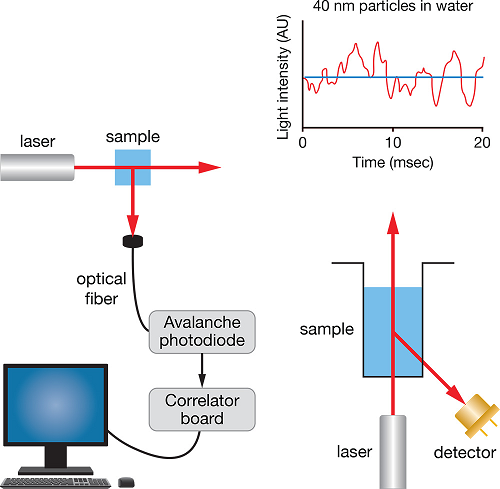
DLS utilizes the time-dependent fluctuations of scattered intensity, which arise from Brownian motion, in order to determine the diffusion constant. The hydrodynamic radius Rh is then calculated directly as described in DLS Theory. Unlike MALS, DLS does not usually require accurate knowledge of sample concentration but does need accurate values of solvent viscosity and temperature.
DLS determines size via the rate of fluctuation of the scattering signal.
Size distributions by on-line (fractionated) DLS
DLS may be measured on-line in SEC or FFF by integrating a WyattQELS™ module into a DAWN, miniDAWN or microDAWN MALS detector, or by transferring light collected in the MALS detector via optical fiber to a batch DLS detector such as the DynaPro™ NanoStar™ or DynaPro™ ZetaStar™. Depending on the collection angle, laser wavelength and flow rate, online DLS can analyze Rh from 1 nm up to ~250 nm.
Applications
- Quantum dots and metallic nanoparticles not amenable to MALS analysis because of size or anomalous refractive index
- Conformation of proteins and protein aggregates
- In conjunction with MALS size determination (Rg), conformations of biopolymers and other heterogeneous macromolecules, for example discriminating between filled and unfilled liposome drug delivery vehicles
Size by batch (unfractionated) DLS
In batch mode (no flow), DLS can measures particles as small as 0.2 nm and as large as 5000 nm in radius. Another valuable feature of batch mode DLS is its ability to extract a coarse size distribution, without any fractionation, by means of regularization analysis. While distributions calculated from batch DLS can only clearly separate populations which differ by about 3x-5x in radius, aggregates and other forms of heterogeneity may be assessed via the peak width, also known as polydispersity.
- Cumulants analysis estimates the polydispersity of a 'monomodal' distribution of particle sizes
- Through NNLS regularization analysis, DLS discriminates between populations of particles that differ in size by at least ~3-5x
Despite the low size resolution, DLS is quite sensitive to shifts in average sizes. The ability to sense size shifts as low as 1-2% is utilized in analyzing protein unfolding at the thermal melting transition, protein-protein interactions assessed via concentration or composition dependence of size, and even binding of lipids or oligonucleotides to nanoparticles.
The DynaPro NanoStar and DynaPro ZetaStar offers batch DLS in high quality quartz microcuvettes or disposable plastic cuvettes, while the DAWN and miniDAWN offer batch DLS in the flow cell ('microbatch') or in a quartz 10 µL microcuvette using the microCuvette™ Adapter Kit.
Applications
- Quick estimates of average size and polydispersity of organic and inorganic nanoparticles
- Rapid quality control of protein expression and purification, testing for large aggregates
- Simple, semi-quantitative assessment of oligomerization or aggregation states
- Protein melting and protein-protein interaction studies

High-throughput sizing by batch DLS
While less sensitive than MALS to low sample concentrations, DLS is also less prone to interference by stray light and so may be carried out under less stringent optical conditions. The DynaPro™ Plate Reader takes advantage of this particular capability to carry out automated DLS measurements directly in situ in industry-standard microwell plates, measuring hundreds or even thousands of samples and sample conditions per day.
Applications
- High-throughput screening of optimal formulation conditions for proteins and biotherapeutic complexes such as virus-like particles, based on criteria that include aggregation and the thermally induced onset temperature of aggregation
- Determination of protein melting temperature as a function of formulation conditions (excipients, pH, etc.)
- Characterization of non-specific protein-protein interactions via the diffusion interaction parameter kD, evaluated from the concentration dependence of the diffusion coefficient

Online DLS
Dynamic Light Scattering Detectors
DynaPro NanoStar / DynaPro ZetaStar - The NanoStar and ZetaStar cuvette-based DLS detectors serve double duty as an on-line DLS detector by installing its optical collection fiber into a Wyatt MALS detector.
WyattQELS - A DLS module which integrates into a DAWN, miniDAWN or microDAWN MALS detector to provide simultaneous DLS measurements in the same scattering volume as the primary MALS measurement.
Software
DYNAMICS - Software for the DynaPro instruments, enabling measurements of size, polydispersity, molar mass, turbidity, and zeta potential. Set-up analysis of kD or A2 and derive parameters such as the melting temperature Tm and the aggregation onset temperature Tagg.
DYNAMICS TOUCH - Touch screen software enabling guided workflows and walk-up measurement setup and data collection. Data quality indicators provide confidence in the results as well as insight to help improve future measurements. Results are displayed right away and can be transferred via network export.
CALYPSO - The CALYPSO software does not analyze DLS data, but can be set to control dilutions or composition gradients delivered to a MALS or Mobius detector and trigger DLS data acquisition by Wyatt's ASTRA or DYNAMICS software packages.

Batch DLS
Batch DLS Instruments
DynaPro Plate Reader - The only commercially available instrument that measures DLS directly in situ in standard 96, 384 or 1536 microwell plates, the non-perturbing DynaPro Plate Reader offers integration with robot liquid handlers and multi-technique plate-based assay protocols. Temperature-controlled over 4 °C to 85 °C. An integrated camera views each well for troubleshooting and diagnostics.
DynaPro NanoStar - With sample volumes as small as 1.25 µL and temperature control spanning -15 °C to +150 °C, the NanoStar goes above and beyond traditional cuvette-based DLS instruments. It offers an optimized static light scattering detector in parallel to the DLS detection system in order to determine true molar mass. The NanoStar does double duty as an online DLS detector by installing its optical collection fiber into a Wyatt MALS detector.
DynaPro™ ZetaStar™ - A manual or automated light scattering instrument for analysis of size distribution, particle concentration, molecular weight, turbidity and zeta potential of proteins, nanomedicines, gene vectors, extracellular vesicles, nanoemulsions and polymers. It uniquely measures DLS and ELS simultaneously and can measure zeta potential in salts solutions greater than physiological conditions.
Calypso - Preparative and sample delivery system and software for carrying out and analyzing automated concentration or composition gradients in a MALS detector. Automates reliable and repeatable time- or composition-dependent size measurements by DLS in a DAWN or miniDAWN equipped with WyattQELS or a NanoStar feed.
microCuvette - Allows static and dynamic light scattering measurements to be made simultaneously in a DAWN using a quartz cuvette, requiring only 10 µL.
Microbatch Kit - Used to inject samples into the flow cell of a MALS detector.
NanoFilter Kit - Makes it easy to filter, recover and refilter precious sample. This is especially useful when your total sample volumes are below 300 µL.
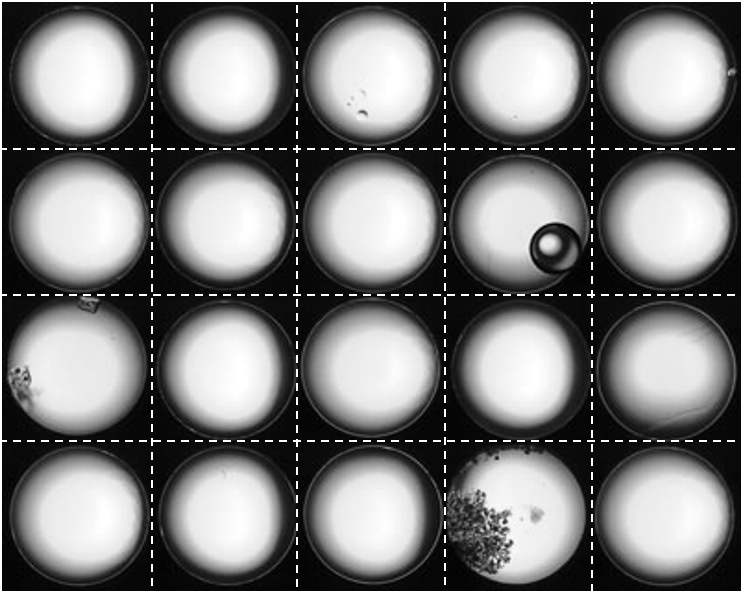
A set of images collected by the onboard camera, showing clean wells and wells containing bubbles, crystals and precipitates.
Size by Viscometry
For polymers, size determination by viscometry is often more appropriate than DLS. Size is determined from the Einstein-Simha equation relating intrinsic viscosity to size and molar mass. Viscometry is more sensitive than DLS to small macromolecules and hence is the go-to technique for polymers and peptides below 10 nm in rg.
Size distributions by intrinsic viscosity + MALS
When polymers are too small to determine size distributions directly by online MALS, the addition of a ViscoStar™ or microViscoStar™ to the SEC instrument stack offers an alternative solution. Hydrodynamic volume is directly related to the product of molar mass and intrinsic viscosity via the Einstein-Simha relation Vh=M[η]/(2.5NA), and hydrodynamic radius may be calculated from the hydrodynamic volume.
Since both intrinsic viscosity and molar mass are determined without assumptions, the SEC-MALS-IV analysis provides an objective estimate of molecular size calculated independently of column interactions.

Viscometry and dRI
Viscometer
ViscoStar - A highly sensitive, on-line differential viscometer used in conjunction with SEC-MALS to determine the size and conformation of all types of biopolymers, synthetic polymers and even proteins and peptides.
The ViscoStar incorporates multiple novel technologies to provide the highest sensitivity, stability and solvent compatibility of any available viscometer for GPC. Its ease-of-use and serviceability make it the perfect companion for Wyatt's DAWN light scattering and Optilab™ refractive index detectors. Temperature controlled from 4 °C to 70 °C.
microViscoStar - Similar to the ViscoStar but designed specifically for use with UHPLC/APC.
Refractive Index Detectors
Optilab - A unique on-line differential refractometer for measuring concentration of any macromolecule, regardless of chromophores. Temperature controlled from 4 °C to 65 °C. The high-concentration option accommodates protein concentration up to 180 mg/mL.
microOptilab™ - The first RI detector specifically designed for use with all UHPLC systems.

Size by FFF
Field-Flow Fractionation Systems
Field-flow fractionation performs versatile separations of macromolecules and nanoparticles ranging from 1 to 1000 nm in diameter and beyond. FFF-MALS-DLS is the ideal means for obtaining distributions of molar mass and size, nanoparticle concentration and extended characterization of conformation and conjugate content.
Eclipse™ - An FFF instrument offering superior performance, reliability and user experience. Supports the Dilution Control Module for enhanced sensitivity, and the SEC switching option for sharing the system between FFF and SEC separation modes.
Mobility EAF4 system - An add-on to an Eclipse which performs electrical/asymmetrical-flow FFF for determining charge and zeta potential distributions.
Eclipse Channels - The Eclipse offers a wide selection of separation channels and membranes to accommodate a variety of analytical and even semi-preparative tasks.
Software
VISION™ - Controls the Eclipse Field-Flow Fractionation (FFF) system. Coupled with ASTRA for data collection and analysis, VISION provides users with a turn-key solution for macromolecular characterization.
Resources
Application Notes
Selected References
Ahmad, A.; Uversky, V. N.; Hong, D.; Fink, A. L. Early events in the fibrillation of monomeric insulin. J. Biol. Chem. 2005, 280, 42669-42675.
Aichmayer, B.; Widermann-Bidlack, F. B.; Gilow, C.; Simmer, J. P.; Yamakoshi, Y.; Emmerling, F.; Margolis, H. C.; Fratzl, P. Amelogenin nanoparticles in suspension: deviations from spherical shape and pH-dependent aggregation. Biomacromolecules 2010, 11, 369-376.
Striegel, A. M. Architecture on the mechanochemical degradation of macromolecules. J. Biochem. Bioph. Meth. 2003, 56, 117-139.
Tarazona, M. P; Saiz, E. Combination of SEC/MALS experimental procedures and theoretical analysis for studying the solution properties of macromolecules. J. Biochem. Bioph. Meth. 2003, 56, 95-116.
Wyatt, P. Submicrometer particle sizing by multiangle light scattering following fractionation. J. Colloid Interf. Sci. 1998, 197, 9-20.
