Dynamic Light Scattering (DLS)
Size distributions measured quickly and easily
Widely used in biochemistry, biotechnology and pharmaceutical development, dynamic light scattering (DLS) is popular wherever the size distributions of nanomaterials need to be measured quickly and easily for a wide range of analytes such as:
- Proteins, peptides, nucleic acids and their aggregates
- Nano-drug delivery systems, e.g., liposomes, lipid nanoparticles (LNPs), micelles, gene vectors and viruses
- Nanoparticles and nanoplastics
- Polymers and hydrogels
- Compound aggregates
Whether measuring directly in microwell plates, in conventional cuvette-based format, automated or online in conjunction with a MALS detector, Wyatt offers instruments and options to fit your needs.
What is DLS?
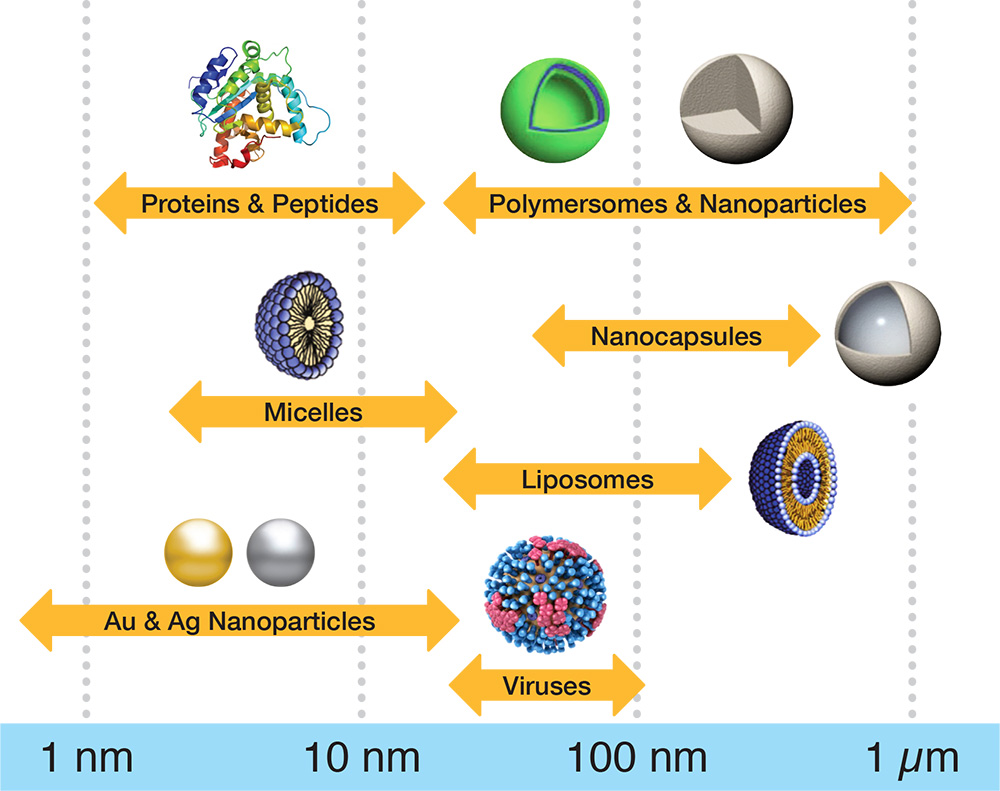
Dynamic light scattering is a powerful, yet easy to use technique to measure the hydrodynamic size and related parameters of macromolecules and nanoparticles in solution, without the need for chromatographic separation. Assess sample properties such as:
- Size and purity of proteins
- Soluble and insoluble aggregates up to 5000 nm for small molecules, bio-macromolecules and nanoparticles
- Particle concentration of nanomedicines
- Thermal, colloidal and chemical stability of protein formulations
- Conformational changes in macromolecules
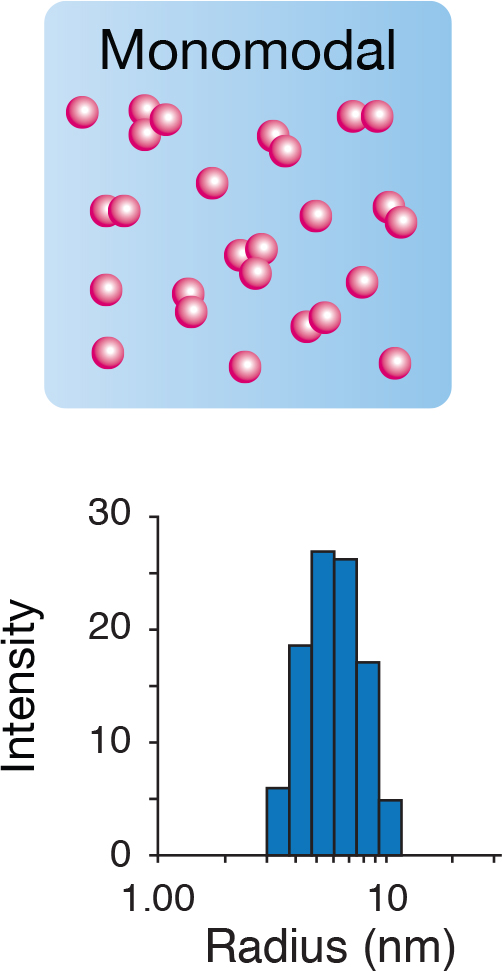
Why DLS?
DLS is an ideal screening tool for biopharmaceutical formulations and other nanomaterials. DLS offers unique benefits such as:
- Fast: get data in less than a minute
- Full nanometer size range: from 0.5 nm to 2.5 µm in a single measurement, with a resolution up to a factor of 3-5 in size.
- Non-destructive and fully recoverable: Samples may be transferred to other assays
- Low sample volume: Only 2 µL in the DynaPro NanoStar using quartz cuvettes, 4 µL in disposable cuvettes or standard microwell plates in the DynaPro Plate Reader
- No shearing or filtering: always measure what’s in your solution
- Versatility: Beyond size and size distributions, measure other properties such as stability, stress-induced aggregation and molecular interactions
- Advanced properties: Measure particle concentration, nonspecific interactions (A2 or B22) and differentiate between conformational changes and aggregation with the static light scattering option in the NanoStar and Plate Reader
- Pre-screening: Ideal to check sample quality before running other expensive and time-consuming biophysical methods such as TEM, AUC, SAXS/SANS, FFF and SEC.
For in-depth characterization of high-resolution size distributions and other physical properties, SEC-MALS and FFF-MALS offer complementary and orthogonal techniques to DLS.
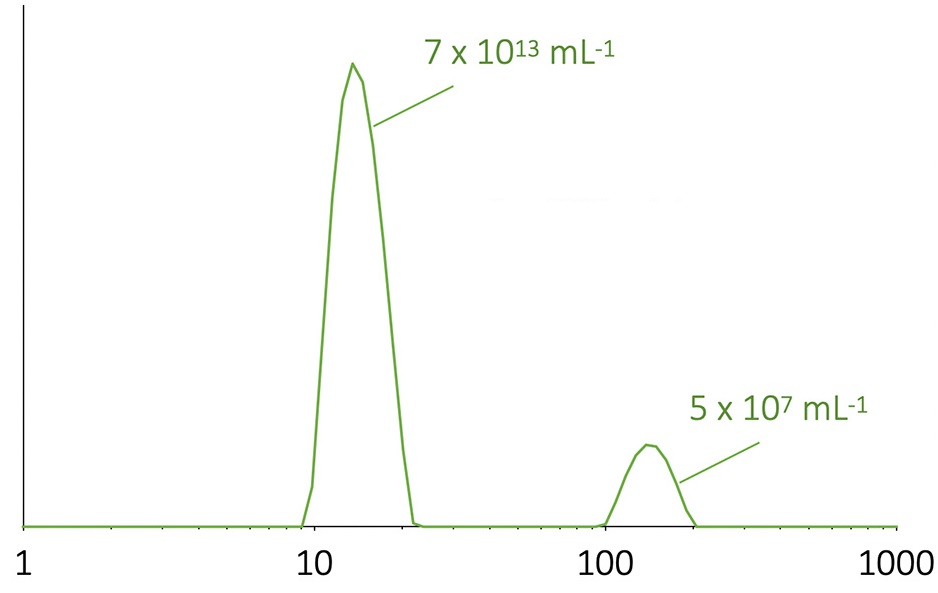
DLS size distribution of a mixture of adeno-associated viruses (AAV) and aggregates. The intensity-weighted distribution, shown, highlights aggregates; the graph may be converted to a mass-weighted distribution, which would indicate that the true aggregate content is relatively much lower. The particle concentration of each population is calculated by combining DLS with static light scattering (SLS).
Who uses DLS?
DLS is used to analyze samples in the nanometer size range, by:
- Biopharmaceutical scientists for characterization and process development of gene therapies, vaccines and biologics such as monoclonal antibodies
- Pharmaceutical technologists for developing nanodrugs such as micelles, liposomes, nanocrystals and polymeric controlled-release particles
- Molecular biology and nanomedicine researchers for studying liposomes, lipid nanoparticles and extracellular vesicles
- Polymer analytical chemists for characterizing biopolymers, thermo-responsive polymers, polymer solubility and polymer latex particles
- Environmental scientists to study nanoplastics, tracer particles and toxic materials in the environment
- Nanoparticle engineers for advanced materials

Versatile technology
While dynamic light scattering instruments are generally used for single samples in batch or standalone configuration, they may also be employed for flow-injection analysis and in conjunction with chromatography and MALS detectors.
With the DynaPro™ Plate Reader, you can even take DLS measurements in situ in industry-standard 96, 384, or 1536 microwell plates with as little as 4 µL of sample!

How does DLS work?
DLS determines the hydrodynamic size of a nanoparticle in solution by measuring the time scale of Brownian motion. The sample is illuminated with a laser beam, and light waves scattered from the particles interfere constructively and destructively as the particles diffuse randomly. The resulting intensity fluctuations in the light scattering detector are analyzed to determine a translational diffusion coefficient, which is converted to hydrodynamic radius via the Stokes-Einstein relationship (see sidebar). The only necessary input parameters are solution viscosity and refractive index.
DLS is a technique that can be readily applied for sizing unknown samples:
- Solvent viscosity and refractive index are generally well-known and tabulated. Moreover, DYNAMICS™ software contains an extensive, built-in solvent library with the most common solvents and buffer systems, including the option to create custom solvents.
- No sample-specific properties such as concentration or composition are required to assess size, size distributions and aggregation.
- Temperature is controlled and continuously measured in the instrument
For advanced measurements, a few more parameters need to be selected or entered into DYNAMICS:
- Particle concentration: input sample refractive index
- kD and A2: input sample concentration
The DLS Theory page contains additional details on the technique, including a video of Brownian motion.
![]()
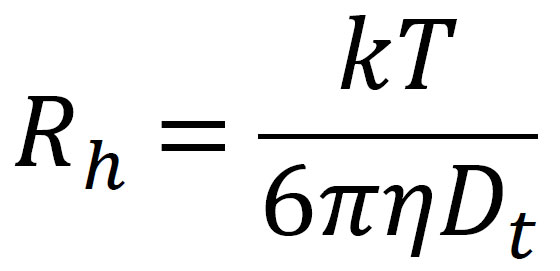
The Stokes- Einstein relationship describes how the hydrodynamic radius Rh is calculated from the diffusion coefficient Dt. T is the temperature (in Kelvin), k is the Boltzman constant and h is the viscosity of the solution.
What are available DLS options and configurations?
Measure directly in microwell plates
High-throughput, automated measurements performed directly in standard 96-, 384- or 1536-well microwell plates reveal new horizons for dynamic light scattering. Some of the high-throughput screening tasks accomplished with the DynaPro Plate Reader include:
- Quantifying the type and degree of aggregation in hundreds of buffer conditions, important in formulation and crystallization studies
- Evaluating the developability of pre-formulations of biotherapeutics
- Formulation development, e.g., assessment of buffer excipients and pH for colloidal stability using the diffusion interaction parameter kD
- Assessing multiple formulations for stability, e.g., freeze-thaw cycles, accelerated aging studies or thermal stability screening using the aggregation temperature Tagg or melting temperature Tm
- Determining the critical aggregation concentration for small molecules such as compound aggregates
- Integration of plate-based testing protocols with other assays in well-plate format

Conventional cuvette-based systems
For easy and convenient measurement of a few samples, all you need is a few microliters of your sample, a disposable or quartz cuvette and a dynamic light scattering instrument such as the DynaPro™ NanoStar™ or DynaPro™ ZetaStar™ with DYNAMICS software for data acquisition and analysis.
- NanoStar has a 90° DLS detector to measure size and size distributions in the range of 0.2 to 1000 nm radius and requires sample volumes as low as 2 µL. In addition, the dedicated 90° SLS detector enables measurement of particle concentration, non-specific interactions, turbidity and molecular weight.
- Mobius has a 163.5° DLS detector to measure size and size distributions up to 2500 nm radius in the flow cell and requires 170 µL of sample. With the quartz cuvette, sample volumes as low as 45 µL may be measured.
- ZetaStar has a 163.5° and 90° DLS detector to measure size and size distributions in the range of 0.2 to 1000 nm radius and requires sample volumes as low as 2 uL. In addition, the dedicated 90 SLS detector enables measurement of particle concentration, non-specific interactions, turbidity, and molecular weight. The PEEK flow cell and disposable cell can both measure DLS simultaneously with ELS data using the back-scatter angle as well.

Integration with SEC-MALS
On-Line DLS is a valuable addition to your SEC-MALS or FFF-MALS systems, providing hydrodynamic size and, in combination with MALS, information about conformation, elution behavior and the shape of larger nanoparticles. Instead of adding another detector in-line, with the associated band broadening, dilution, signal misalignment and loss of resolution, Wyatt's innovative solution is to share the MALS laser and flow cell with the DLS detector.
A WyattQELS™ DLS module may be embedded within a MALS instrument, or a NanoStar or ZetaStar can be coupled to a MALS detector employing an optical fiber. ASTRA™ chromatography software connects to both instruments to acquire data simultaneously, analyzing and reporting the individual information on size (rms radius by MALS and hydrodynamic radius by DLS) and molecular weight to give insights on molecular conformation and chromatography.

Robotic interface
Advanced lab automation is available via the DYNAMICS Automation API. Using the DYNAMICS Automation API to operate DYNAMICS remotely, the DynaPro Plate Reader may be integrated within a robotic workflow. This feature is also available for ZetaStar or NanoStar for an automated workflow or experimental apparatus.
Measurement automation
DYNAMICS and HPLC Connect software pair with the ZetaStar to enable automated sample handling with a Waters Autosampler. The autosampler and HPLC pump are controlled directly by DYNAMICS to flush the system, inject sample, perform the measurement, and repeat with the next sample. The system may even be programmed to automatically prepare samples with a series of buffer conditions.

Key DLS applications
DLS is a key characterization technique for sizing, assessing aggregations and measuring stability in nanomedicines, proteins and related bio-macromolecules as well as nanoparticles. We’ve listed some representative applications with application notes and on-demand webinar resources below. Our searchable Bibliography, containing thousands of references to peer-reviewed articles citing Wyatt instruments, is an excellent resource for researching your specific DLS applications.

Proteins and bio-macromolecules
Formulations and aggregation
Assessing a drug candidate’s suitability early in the discovery and development stages is essential for minimizing the risk of costly downstream failure. DLS delivers essential insight into biopharmaceutical candidates and formulations through pre-formulation studies, early and late-stage formulations as well as quality control.
Explore the versatility of dynamic light scattering in our white paper WP5010: Screening Developability and Pre-Formulation of Biotherapeutics with High-Throughput Dynamic Light Scattering (HT–DLS), and our publication list PL5011: Key publications on high-throughput screening of therapeutic protein formulations by light scattering.
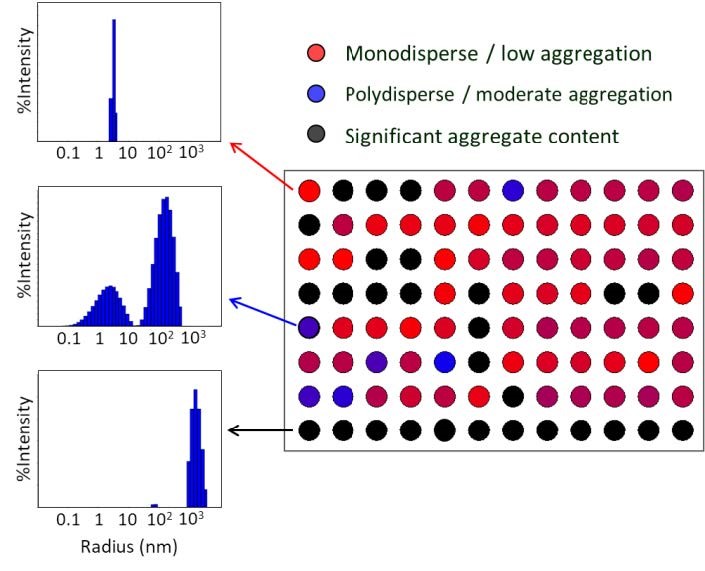
The heat map display in DYNAMICS™ may be programmed to differentiate size distributions and visualize at a glance different degrees of aggregation. Data courtesy Sabin Vaccine Institute.
Colloidal and thermal stability
The rational biophysical characterization of therapeutic protein candidates and their formulations can greatly improve the early-stage development process of new biologics. DLS is crucial to understanding the thermal and long-term stability of therapeutically relevant proteins in different formulation conditions.
View the webinars Rational use of dynamic light scattering for the characterization of cytokines, bispecific antibody fusion proteins and binary antibody mixtures and Predicting stability of antibody therapeutics by unfolded-state studies with high-throughput dynamic light scattering (HT-DLS) for more information.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are important biotherapeutic candidates that combine highly potent cytotoxic drugs with monoclonal antibodies (mAb) for targeted drug delivery in the treatment of cancer or neurodegenerative disorders.
Read the application note AN5005: Characterization and Formulation Screening of mAb and Antibody-Drug Conjugates (ADCs) by High-throughput DLS to learn how the DynaPro Plate Reader is an effective tool for rapid formulation screening.
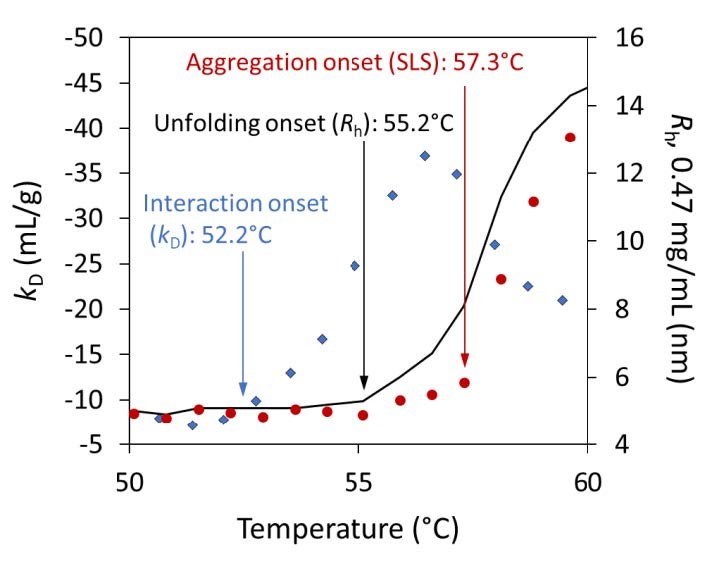
The diffusion interaction parameter, kD , decreases prior to the point of aggregation as protein molecules begin to unfold. The onset of aggregation is measured by SLS. Blue diamonds: kD values; black line: Rh values; red circles: SLS values. Three distinct transition temperatures are evident. The kD scale has been inverted for clarity
Nanomedicines
Small viral vectors and virus-like particles
Viruses like AAV, adenovirus and lentivirus, as well as virus-like particles may be characterized by DLS for critical quality attributes such as size, aggregate content, thermal stability and total viral particle concentration.
To learn more, view AN5007: Characterization of AAV-based viral vectors by DynaPro DLS/SLS instruments.
Liposomes
Liposomes are one of the most frequently used nanocarriers to encapsulate small molecule drugs. FFF-MALS-DLS is a powerful tool for characterizing the size, particle concentration and structure of large nanoparticles to distinguish between filled and empty liposomes.
View AN2601: Liposome Size, Concentration and Structural Characterization by FFF-MALS-DLS.
Lipid Nanoparticles
Lipid nanoparticles are of increasing importance for small molecule drug delivery, and more recently, nucleic acid delivery. Characterization needs include determination of size, titer, stability and aggregation behavior.
Learn how DLS, SEC-MALS and FFF-MALS can be used to characterize this important class of nanomedicines in our recent webinar Size, Concentration, Payload and Quality: Comprehensive Characterization of LNP-RNA Therapeutics by Light Scattering.
Nanocapsules
Protein-based nanocapsules are highly interesting for stimuli-responsive targeted drug delivery, as protein properties can directly influence particle stability and release behavior.
Learn how properties of nanodispersions, such as dispersion stability, agglomeration and temperature stability based on particle size analysis can be explored in AN4001: Organic-phase encapsulation in protein-based nanocapsules studied by DLS.

Colloids and Nanoparticles
Environmental nanoparticles
Characterization of nanoparticle size and distribution in biological environments and understanding the parameters that affect them are imperative to accurately assessing nanoparticle toxicity. Delivering nanoparticles in a well-dispersed form is necessary for accurate assessment of their toxicity.
Read AN5001: TiO2 nanoparticle dispersions in cell culture media characterized by high-throughput DLS to learn more.
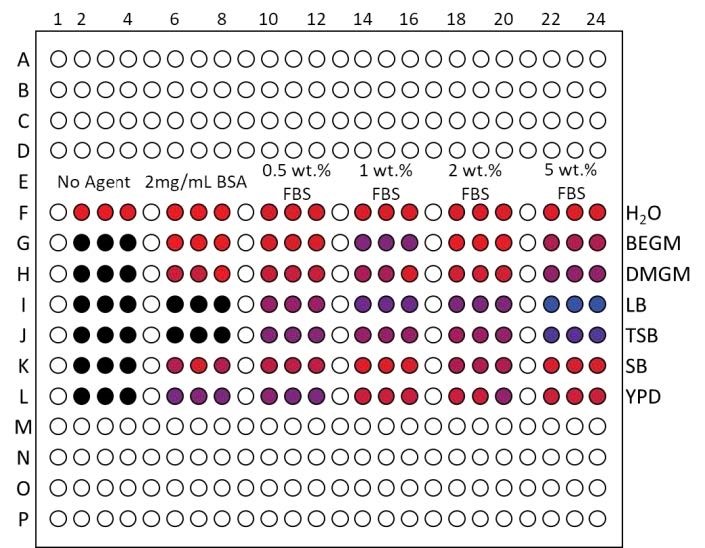
SpectralView display of TiO2 nanoparticle dispersion in water and six different cell culture media (BEGM, DMEM, LB, TSB, SD, and YPD) suggests severe agglomeration in all media without dispersing agents, improved dispersion in most media using 2 mg/mL BSA, and the best dispersion using ≥1 wt.% FBS.
Drug nanosuspensions
Drug nanosuspensions are a promising approach to formulation and consist of crystalline drug particles that are generally <400 nm in diameter suspended in aqueous solution. Nanocrystalline particles have a large surface area relative to their overall sizes, which results in a dramatically increased rate of dissolution, improved absorption rates and increased bioavailability.
Explore AN5002: High-Throughput Tools for Optimizing Drug Nanosuspensions to learn how the DynaPro Plate Reader is key in optimizing these formulations in early drug discovery.
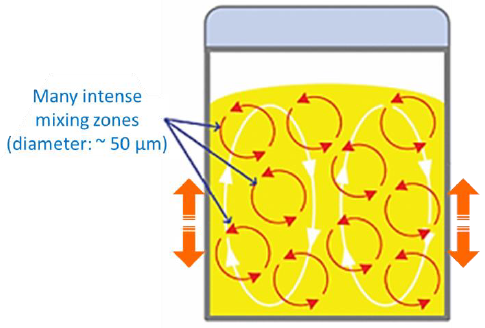
Acoustic resonance milling effectively reduces solid compound to sub-micron particles, rapidly and in a small volume.
Dynamic light scattering – measuring with ease
DLS an essential characterization tool for proteins, nanomedicines and other nanoparticles to screen for properties such as size, aggregation, stability and interactions. The technique’s versatility and simplicity has contributed significantly to its popularity.
How do I use DLS?
Running a DLS experiment is straightforward. A typical DLS experiment comprises the following steps:
- Sample preparation (minimal, may include filtration or dilution)
- Load sample into a cuvette or an array of samples into a microwell plate
- Place the cuvette or microwell plate into your instrument
- Collect data manually or using automation (see below)
- Analyze data

Data collection may be automated in several ways:
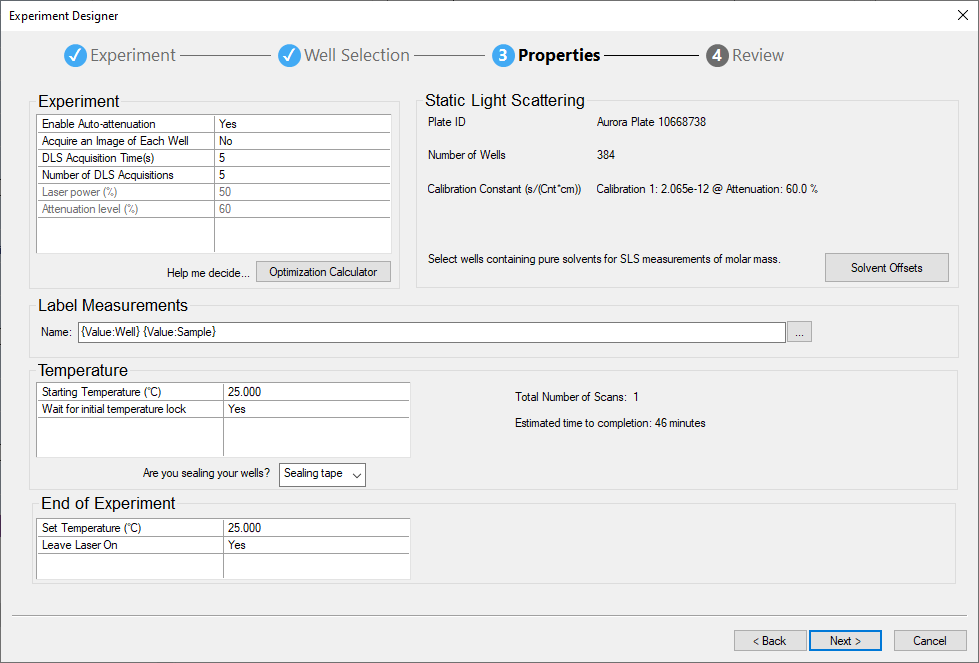
- Fast screening: Experiment Designer - Perform full or partial plate scans, thermal or time stability studies by simply selecting a range of wells or single wells for measurements with a mouse-click
- Maximum flexibility: Event Scheduler - Design any standard operating procedure, be it a time or thermal sequence, autosampler automation, exploration of experimental parameters or any combination thereof.
- Fully automatic: Robotic integration - Use DYNAMICS API to interface with a liquid handling robotic system to automatically load or unload wellplates and run, process, save and export experiments.
Sample and instrument preparation
Instrument setup is minimal: Just switch on your instrument and let it equilibrate to the correct temperature. Some precautions must be taken when preparing samples as light scattering is sensitive to particulates. A few simple steps will ensure successful analysis:
- Wyatt disposable cuvettes are individually packed in a clean room and should remain wrapped until use. Quartz cuvettes should be capped when stored.
- Buffers should be filtered to 0.02 µm or 0.1 µm. Measuring pure buffer is recommended as a control.
- Samples should be centrifuged or filtered in a way that does not remove any analytes of interest.

DYNAMICS
Data collection is straightforward: Click the Start button to run single measurements or use the experiment designer or event scheduler described above to collect data automatically. All results are acquired in real-time and analyzed automatically. View your results in a data table or graphically. All original data are accessible and may be reprocessed at any time. Some of the analysis features are:
- Size, size distributions and molar mass
- Zeta potential and charge
- Particle concentration of biopharmaceuticals
- Thermal stability such as Tonset, Tagg and Tm, as well as average size at transition points
- Formulation colloidal stability via the diffusion interaction parameter kD and second virial coefficient A2
- Solution viscosity
- General parametric analysis
For those in GMP/GLP-regulated environments, the DYNAMICS Security Pack add-on enables 21 CFR Part 11 compliance including full audit trails and electronic signatures.
Wyatt Technology provides several avenues for supporting novice and advanced users, including a full set of tutorials, training materials and technical notes in the online Wyatt Support Center, phone support and Light Scattering University. Site visits may be arranged for IQ/OQ, service and preventive maintenance as well as group training.
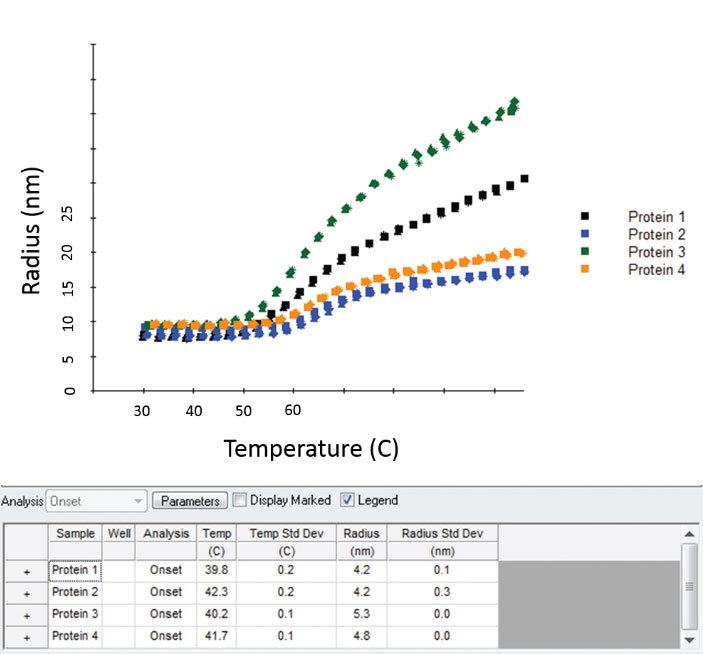
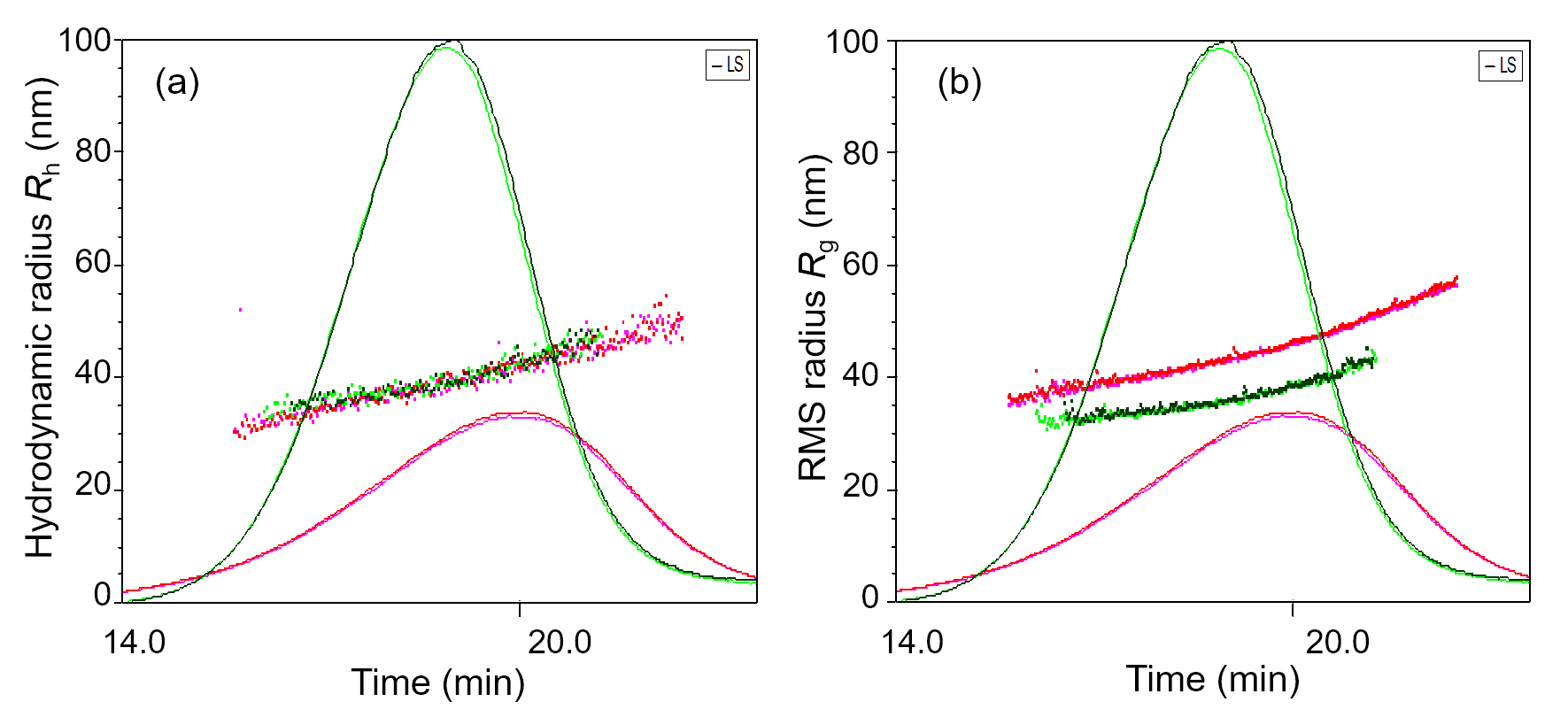
Melting/aggregation curve data and analyses for four proteins measured simultaneously, in triplicate, on the DynaPro Plate Reader.

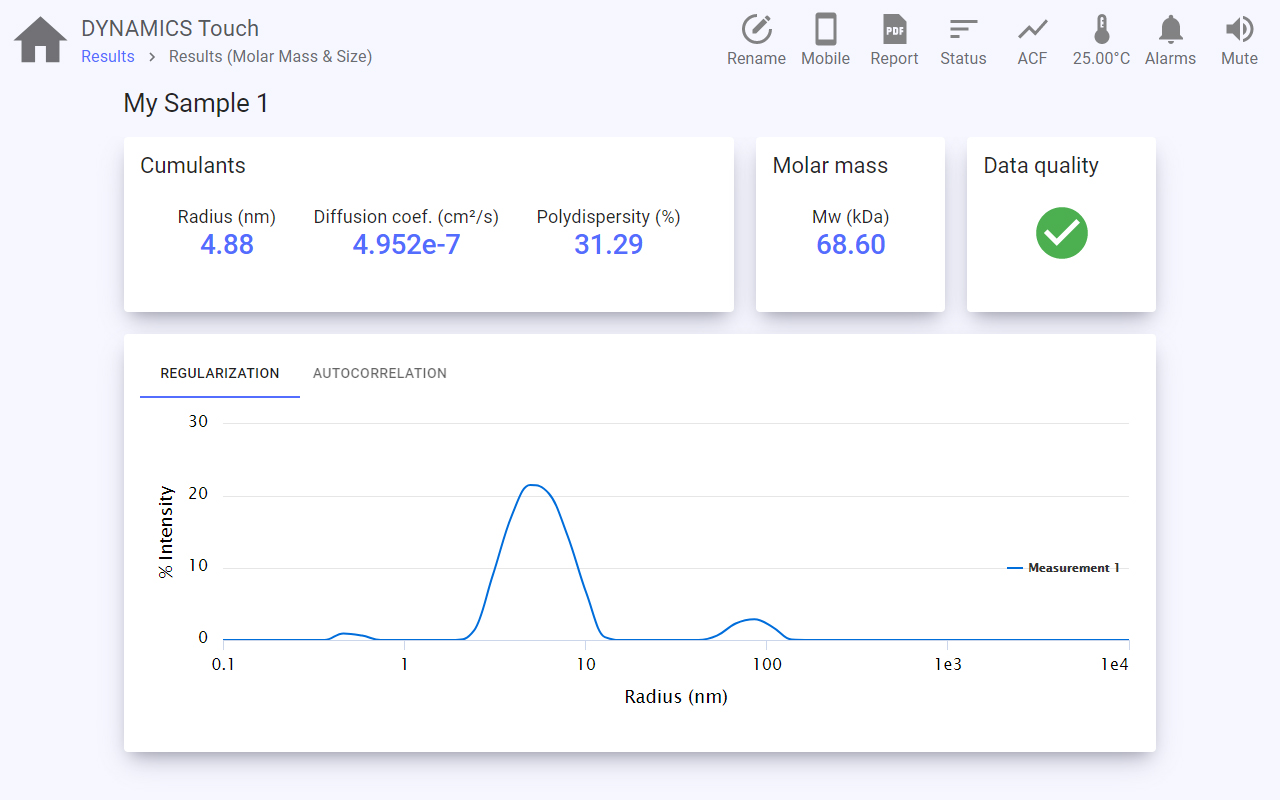
DYNAMICS Touch
DYNAMICS™ Touch™ is the on-board application for quick and easy walk-up measurements of size and molar mass on the DynaPro™ Plate Reader, NanoStar™ and DynaPro™ ZetaStar™.
The intuitive touch-screen interface guides you through each step of your measurement. Load the cuvette with your sample into the instrument and:
1. Choose what you wish to measure
2. Input parameters and collect data
Step by step, specify the:
- Cuvette (quartz, disposable, or generic)
- Sample (dn/dc, concentration, A2)
- Solvent (predefined or custom)
- Temperature
- Number of replicates
- Next, simply press ‘play’ to start the measurement.
You can also manage commonly used cuvettes, custom solvents, and other key parameters directly in DYNAMICS Touch and use presets to streamline your workflow and ensure all users are performing the same analyses.
3. Examine and share your results
All results are analyzed automatically and presented to the user directly in DYNAMICS Touch at the end of each measurement. The automatic data quality assessment allows even first-time users to understand their results and identify any issues with their samples.
The resulting experiment data files and reports can be downloaded to a computer and shared or further analyzed in DYNAMICS. The PDF report may even be viewed immediately on your mobile device, network security permitting.
Unique instrumentation for demanding research
Developed and built entirely in-house at Wyatt’s Santa Barbara headquarters, all of these products incorporate state-of-the-art technology combined with rigorous quality assurance practices. Their rich touch screen displays ensure access to all the control functions, data and diagnostics needed for smooth operation and rapid troubleshooting of samples or instrumentation, should the need arise.

Dynamic Light Scattering/Electrophoretic Light Scattering Instruments
DynaPro™ Plate Reader - The only commercially available instrument that measures DLS and SLS for size molar mass and peak-resolved particle concentration, directly in situ in standard 96, 384 or 1536 microwell plates. Measures second virial coefficient A2 and diffusion interaction parameter kD. The non-perturbing DynaPro Plate Reader offers integration with robot liquid handlers and multi-technique plate-based assay protocols. Temperature-controlled from 4 °C to 85 °C. An integrated camera views each well to observe precipitation or turbidity as well as troubleshooting and diagnostics.

DynaPro™ ZetaStar™ - A manual or automated light scattering instrument for analysis of size distribution, particle concentration, molecular weight, turbidity and zeta potential of proteins, nanomedicines, gene vectors, extracellular vesicles, nanoemulsions and polymers. It uniquely measures DLS and ELS simultaneously and can measure zeta potential in salts solutions greater than physiological conditions.
WyattQELS™ - An online dynamic light scattering module that may be embedded into any MALS instrument to provide integrated and simultaneous DLS data.

DynaPro™ NanoStar™ - With sample volumes as small as 2 µL and temperature control spanning -10 °C to +120 °C, NanoStar goes above and beyond traditional cuvette-based DLS instruments. It offers an optimized static light scattering detector in parallel to the DLS detection system to determine absolute molar mass, turbidity and in conjunction with DLS, particle concentration per peak.
The intuitive DYNAMICS™ Touch™ on-board app, running on the NanoStar’s generous touch screen, enables walk-up operation and basic measurements even by DLS novices. Extended and more comprehensive analyses are carried out in DYNAMICS, running on a standard PC.

Software
DYNAMICS™ - Versatile software for size, size distributions, particle concentration, zeta potential and charge measurements, combining flexibility and intelligence with rigorous calculations and advanced analysis. DYNAMICS offers an optional 21 CFR Part 11 compliant security and database package for GMP and GLP laboratories.
DYNAMICS Touch – measure and analyze DLS, ELS, and SLS data directly on the ZetaStar touch screen with the on-board app.

ASTRA™ - Our comprehensive software solution for MALS and DLS analysis in chromatography of field-flow fractionation. ASTRA is available in a 21 CFR Part 11 compliant version and offers additional options and advanced analyses.

Introducing the DynaPro™ ZetaStar™
Introducing the DynaPro™ NanoStar™ II
Introducing the DynaPro Plate Reader 4
Transforming automated dynamic and static light scattering in microwell plates
Explore DLS
Below you will find a partial list of the resources available on our website where you can learn more about DLS and how it is used by scientists around the world, in academia, industry and government labs for advanced macromolecular and nanoparticle characterization.
Webinars
On-demand webinars presenting the theory and applications of DLS are available for unrestricted viewing on the DLS Webinars page. Listed below are some of the most popular:
Biotherapeutics
- Virtual User Meeting – Screening for stability: Characterizing formulations with DLS
- Rational use of dynamic light scattering for the characterization of cytokines, bispecific antibody fusion proteins and binary antibody mixtures
- Understanding Monoclonal Antibody Unfolding and Aggregation
- High-throughput Analytics for Formulation and Process Development of Bio- and Nano-therapeutics
Nanoparticles
- Size, Concentration, Payload and Quality: Comprehensive Characterization of LNP-RNA Therapeutics by Light Scattering
- Investigating Hydrogel Microparticles with Light Scattering and Microscopy Tools
- Virtual User Meeting – Viral Vectors and Bio-Nanoparticles
- Novel Ways of Screening Colloidal Nanoparticles Under Preclinical-relevant Conditions
Proteins
Application Notes
Application notes highlighting the use of DLS are available for unrestricted viewing on the DLS Application Notes page. Listed below are some of the most popular:
Biotherapeutics
- WP5010: Screening Developability and Pre-Formulation of Biotherapeutics with HT-DLS
- AN5005: Characterization and Formulation Screening of mAb and Antibody-Drug Conjugates (ADCs) by High-throughput DLS
- PL5011: Key publications on high-throughput screening of therapeutic protein formulations by light scattering
Nanoparticles
- AN5001: TiO2 nanoparticle dispersions in cell culture media
- AN5002: High-Throughput Tools for Optimizing Drug Nanosuspensions
- WP9001: Overcoming the Challenges of Nanoparticle Characterization with a Light Scattering Toolkit
Proteins
- AN5006: Probing the aggregation rate of Tau protein with automated DLS and SLS
- WP5003: Automated dynamic and static light scattering in microwell plates for fast, productive development of biologics
- AN4001: Organic-phase encapsulation in protein-based nanocapsules studied by DLS
- AN7301: Glycoprotein by SEC-MALS-QELS
Theory
Please see the following page for details on DLS theory: Understanding Dynamic Light Scattering Theory.
These pages provide additional information on specific analyses performed in DLS:
Bibliography
An extensive searchable bibliography of publications citing Wyatt DLS instruments is available at www.wyatt.com/Bibliography. Just open the Advanced Search and click the Dynamic Light Scattering box, then enter your search terms below.
Technical Notes
Wyatt offers its customers comprehensive online support via the Wyatt Support Center, including many technical notes that can help make the most of DLS experiments. If you are a customer and do not have access, please request an account on the Support Log-in page.
At Wyatt, it’s personal
Your success is ours! From the time you purchase a Wyatt instrument, we are there for you every step of the way and give you more than just an instrument warranty. Wyatt provides a full suite of support offerings to help you make the most of your investment. Most importantly, you get unrivaled personal attention to ensure success and productivity.

On-site installation and training
With every instrument purchase, we offer an on-site installation and familiarization visit at your laboratory to ensure proper instrument set-up and function. We also offer customized on-site training tailored to your specific needs. These additional services may be especially desirable if a large group of staff members wants to receive instruction.

First-year service and support
All new Wyatt instruments come with a full year of unlimited telephone and e-mail support as well as our standard full-coverage warranty during the first year. Our congenial staff of Ph.D. scientists and expert technicians provides both technical and application assistance. We will show you how to analyze your data or set up instrument communications by remotely accessing your PC.
After the first year, annual service plans are available for continuing, unlimited telephone support, e-mail support and instrument service. Benefits of the service plans include annual preventive maintenance, on-site calibration, re-qualification, and discounts on parts and labor for repairs. Learn more about service plans.
The specifics of first-year service as well as continuing support may vary by region. Please contact your local Wyatt representative for more information.

IQ/OQ and compliance
Wyatt Technology offers a complete compliance program including documentation and on-site validation for all of its instruments. We provide the resources and tools necessary to ensure compliance. We’ve worked extensively with our partners in compliant environments to build up a robust set of documents and services. Wyatt instruments and software are used in GMP environments at pharmaceutical, biotech, and other regulated industries around the world. Learn more about our Compliance program.

Light Scattering University
Our flagship training program Light Scattering University® (LSU) is included with every purchase of a light scattering instrument. In the 2, 3 or 4 day course—depending on which system is purchased—LSU students discover advanced data processing methods and alternative analytical tools they may not be familiar with. The students also learn how their MALS and DLS data complements information from other techniques they are using in the lab. Advanced classes are offered for LSU graduates on more detailed techniques and topics.
During LSU our students meet Dr. Philip Wyatt, Founder and Chairman of the Board who gives an acclaimed Light Scattering Instrument Museum tour. Students also meet those who invent, build, service and support the instruments, so they know the team that will support them when back in the lab.
An LSU credit, inclusive of airfare, accommodations and most meals, is included for North American customers who purchase one of our light scattering instruments. While you are here, we work you hard but feed you well—at a variety of Santa Barbara restaurants!
Learn more here.

Service and Support Plans
At Wyatt, we understand that downtime and loss of productivity is critical and have developed comprehensive service offerings — our Platinum, Gold and Silver Service Plans — specifically designed to make preventative maintenance and instrument repair requests quick, smooth and hassle-free. These plans are available on an annual basis after the first-year instrument warranty expires and offer priority phone and e-mail support in addition to instrument service.
The Platinum and Gold Service Plans offer comprehensive on-site preventive maintenance, repair services and software updates. A highly trained Wyatt Field Service Engineer will come to your facility and perform annual or semi-annual preventative maintenance, calibration and optional re-qualification checks. We also offer loaner units should an instrument require factory repair. With our Silver Service Plan, you can expect full instrument calibration and quality control testing, priority service including parts, labor, shipping, as well as the same comprehensive, first-priority technical and application support by phone, email, and screen sharing sessions as offered with the Platinum and Gold Service Plans.
Learn more here.
![]()
Web-based support
Our Customer Support Center contains a wealth of useful resources on everything related to your Wyatt light scattering instruments, software and applications:
- Software updates and bug fixes
- Technical Notes on how to connect and work with your instruments and software
- Video tutorials, software tutorials and educational webinars
- Learning Resource section with New User Guides
- Reference materials such as User's Manuals, Certificates of Analysis for Wyatt-supplied standards, CE and TUV declarations
Register or log in to the Support Center here.
We're Here to Help
Phone number +1 805-681-9009 option 4
Business hours: M-F 8 a.m. - 5 p.m. Pacific
Consulting
By special arrangement, we can provide extended on-site consulting services by an Applications Scientist to set up methods and run samples on your instruments in your lab. This avoids the need to send samples off-site for analysis and allows for intimate, real-time consultations with the best experts available. Availability may be limited to certain geographical regions.
If you would like to have our lab staff analyze samples for you and provide consultations, explore Wyatt's in-house Sample Analysis services.

Wyatt Technology Store
With our online store, world-wide customers can search for parts, consumables and accessories and view product images and part numbers. The store is categorized by Wyatt product families making it easy to know which parts are compatible with your instrument. US and Canadian customers can register for an account and order parts and accessories with either a purchase order or a credit card.
Browse our most popular consumables, review your order history and easily place repeat orders online. If you have questions on which Waters™ Wyatt protein SEC column is best for your application, check out the friendly SEC Columns Guide which will interactively guide you to the recommended column. In addition to ordering parts and accessories, you can also inquire about our training courses, IQ/OQ validation, and service plans.
Visit our online store here.