Introduction to Light Scattering Theory
Light scattering comprises a versatile suite of non-invasive techniques for characterizing macromolecules and a wide range of particles in solution. In contrast to most methods for characterization, it does not require outside calibration standards. In this sense it is an absolute measurement.
Wyatt instruments make two different types of light scattering measurements for absolute molecular characterization:
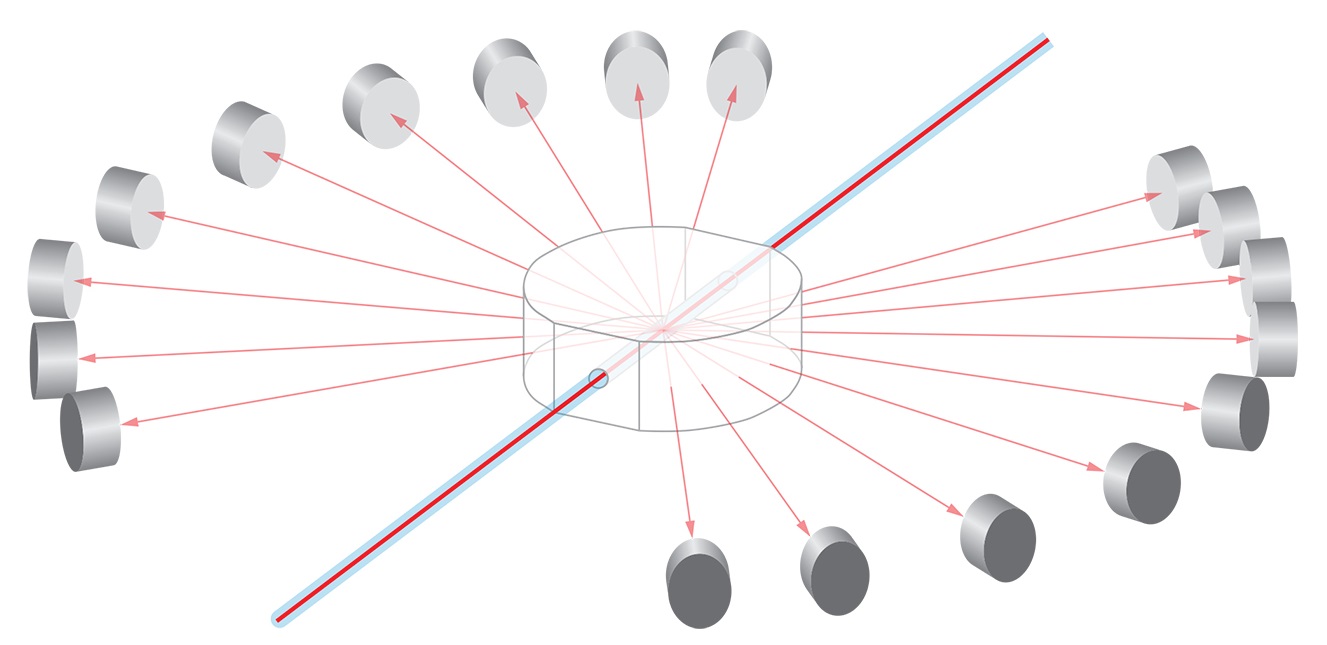
- Multi-Angle Light Scattering (MALS), Static Light Scattering (SLS) or Classical Light Scattering - In MALS the intensity of the scattered light is measured as a function of angle. For macromolecules much smaller than the wavelength of the incident light, the data are analyzed to determine the molar mass or molecular weight Mw and rms radius Rg. For certain classes of particles having sizes in the vicinity of the wavelength of the incident light, MALS can help determine shape and structure.
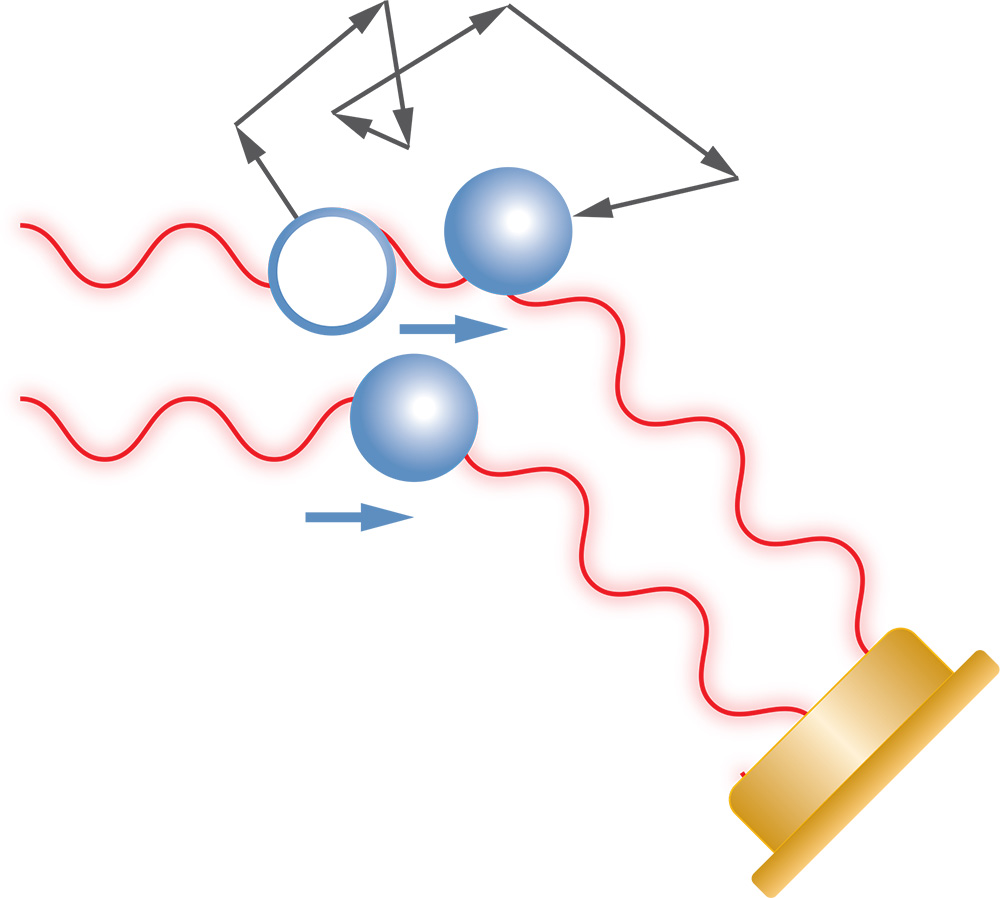
Via the concentration dependence of the scattered intensity, it is possible to quantify molecular interactions as the second virial coefficient (A2, for non-specific interactions) or equilibrium dissociation constant (KD, for non-specific interactions). - Dynamic Light Scattering (DLS) or Quasi-elastic Light Scattering (QELS) - In a DLS measurement, time-dependent fluctuations in the scattered light signal are measured using a fast photon counter. DLS measurements can determine the diffusion coefficient of macromolecules or particles, from which the hydrodynamic radius is calculated.
Light scattering is a technique that can be applied in either batch or chromatography mode. Each technique has its advantages and disadvantages for characterizing different properties of the analyte. Read the Solutions section to better understand how different techniques are applied to various characterization challenges.
Why Light Scattering?
Although absolute molecular weights can also be determined via mass spectrometry, membrane osmometry, and sedimentation equilibrium (analytical centrifugation), only light scattering covers so broad a range of macromolecules including their oligomeric states. Most importantly, light scattering permits measurement of the solution properties of macromolecules. While a sedimentation equilibrium run may require 72 hours, a size exclusion chromatography/light scattering study may be completed in well under an hour, and a batch mode analysis in a few minutes. These comparatively short run times coupled with the absolute determination of molar mass, size, and A2 make light scattering the method of choice for accurate and fast macromolecular characterization.
Chromatography Mode
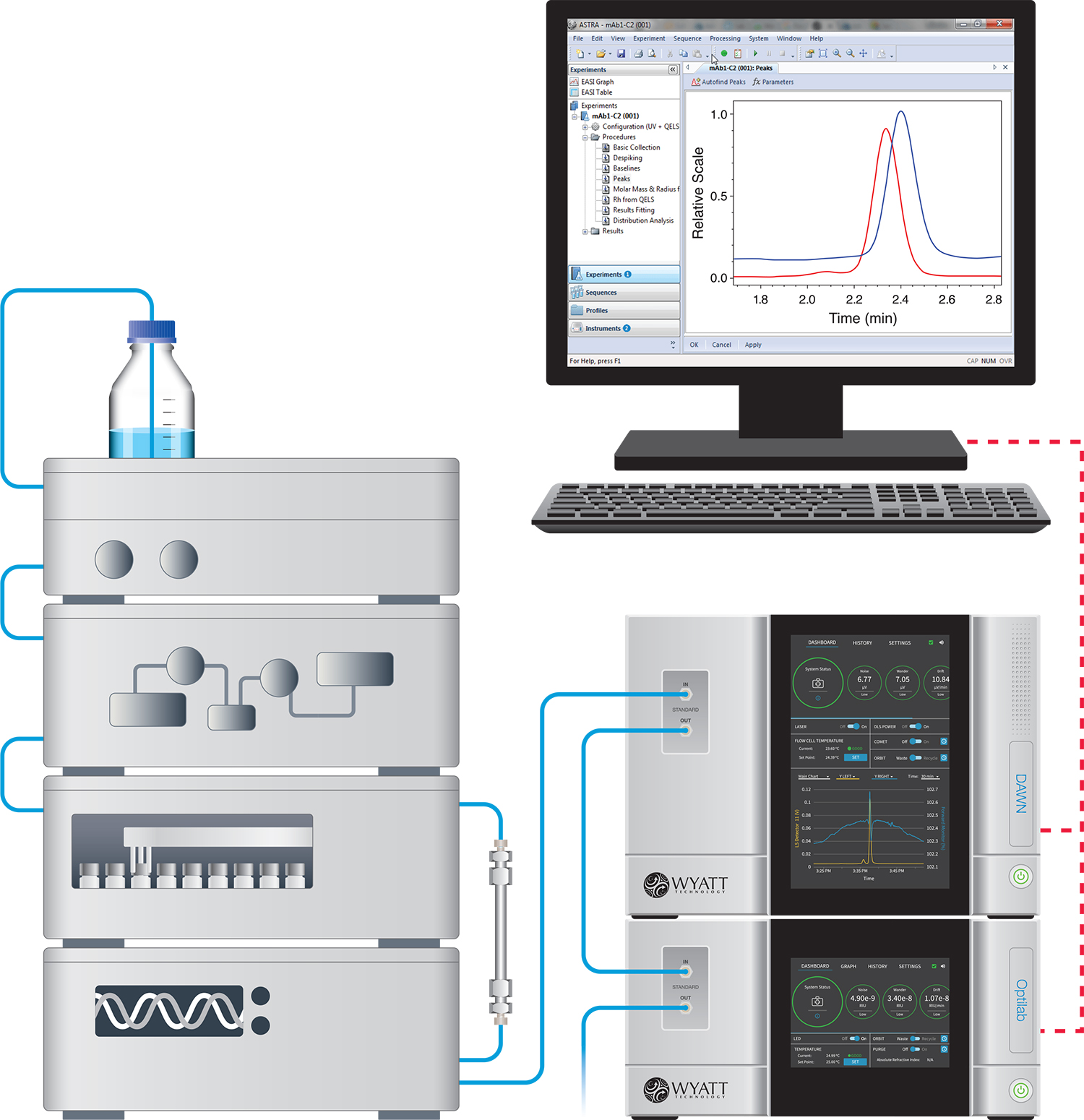
A chromatography-mode light scattering experiment couples a Multi-Angle static Light Scattering (MALS) detector, such as the DAWN™ or miniDAWN™, with a fractionation device that separates macromolecules based on their physical properties. The most common fractionation method used with MALS is HPLC size exclusion chromatography (SEC). SEC-MALS provides accurate distributions of molar mass and size (root mean square radius Rg), as opposed to:
- Standard SEC, which depends on column calibration by reference standards.
- Batch (cuvette) mode MALS, where the measured quantities are averaged over all masses and sizes present in the sample.
This type of arrangement has many advantages over traditional column calibration methods. Since the light scattering and concentration are measured for each eluting fraction, the molar mass and size can be determined independently of the elution position. This is particularly important for species with non-globular shapes or that interact with a SEC column; such species typically do not elute in a manner that might be described by a set of column calibration standards.
For light scattering in chromatography mode, however, non-standard column interaction is no problem, since the absolute molar mass and size are determined for each eluting fraction. Since it is not necessary to make any assumptions inherent with the use of calibration standards, it is possible to completely characterize the distributions derived from separation techniques such as ion exchange or reverse phase chromatography. There are no means to calibrate these techniques by traditional methods.
Another powerful separation technique often used in conjunction with MALS is Field-Flow Fractionation (FFF) which performs separations over a very wide dynamic range. FFF works without a packed column, by means of a channel. The Eclipse™ controls the flows precisely to obtain versatile and robust separations. FFF-MALS characterizes solutes and suspensions from small macromolecules up to micron-sized nanoparticles.
In a typical SEC-MALS or FFF-MALS measurement, the light scattering detector and a concentration detector are connected in series after the fractionation device. The concentration detector is usually a differential refractometer (dRI) such as an Optilab™ or an ultraviolet absorption (UV) detector.
In addition to measuring molar masses and root-mean-square radii via MALS, it may be desirable to determine hydrodynamic radi as well. This is accomplished by adding to the MALS detector a dynamic light scattering (DLS) detection module such as a WyattQELS or the DynaPro™ NanoStar™ external fiber for measuring hydrodynamic radii Rh. SEC-DLS and FFF-DLS size measurements range from < 1 nm up to > 250 nm.
Alternatively, a differential viscometer such as the ViscoStar™ may be connected in series with the MALS and dRI detectors to determine intrinsic viscosity, which can also be related to Rh. The relationship between molar mass and size as determined by DLS or viscometry is indicative of molecular conformation.
Batch Mode
On occasion, it is necessary or desirable to make a light scattering measurement of an unfractionated sample. For example, the time-dependent or concentration-dependent properties of a sample might be required. Such measurements are collectively termed batch measurements, and are made by either injecting a sample aliquot directly into the flow cell, or by introducing the sample in a container such as the microCuvette™ into a DAWN™ MALS detector.
The properties that may be determined in a batch light scattering measurement depend on the measurement technique. In multi-angle (static) light scattering, the presence of species with different masses means that the measured quantities are averaged quantities. For example, the molar mass is a weight-averaged molar mass, and the size is averaged over a higher moment of the mass distribution. However, if batch measurements are made at multiple concentrations, it is possible to determine the second virial coefficient A2 to quanitfy the solute-solute or solute-solvent interaction – very useful information in studies such as protein formulation or crystallography.
Batch measurements of DLS (Dynamic Light Scattering) determine semi-quantitative aspects of the hydrodynamic radius distribution. Using analysis techniques such as regularization (see ASTRA™ software), batch DLS data can reveal the distribution of sizes for broadly polydisperse samples (i.e., over several orders of magnitude), and even differentiate between populations if the size difference is greater than a factor of three to five in radius.
Although chromatography mode is in general the best method for characterizing molar mass and size distributions in a sample, the batch mode is useful in those instances where the macromolecules cannot be easily separated by size exclusion chromatography, or the time or concentration-dependent properties of the sample are of interest. The latter might include studies of phenomena such as protein self-assembly, measurement of concentration-dependent aggregation, etc. Concentration-dependent and time-dependent studies on unfractionated samples may be automated and analyzed using the Calypso™ composition-gradient system and CALYPSO™ software. For more details on the theory behind characterization of interactions with MALS, see Understanding CG-MALS.

Some History, and More
A historical exposition of the evolution of light scattering theory along with the impact of excluded volume interactions and hyperbranching has kindly been provided by Prof. Walter Burchard. Click here to download.
Acknowledgements
Wyatt Technology Corporation is grateful to the following individuals for the use of materials and graphics in this theory section:
- Dr. Ewa Folta-Stogniew at the Yale School of Medicine. For examples of light scattering theory, analysis, applications, and example results, please visit her light scattering website.
- Dr. Eric R. Weeks in the Department of Physics at Emory University. Dr. Weeks provided the Brownian motion animation in the DLS theory section. For information about his research and to view more fascinating pictures, please visit his website.