AN3005: Evaluation binding of individual and combined domains in the bacterial flagellar motor complex by CG-MALS
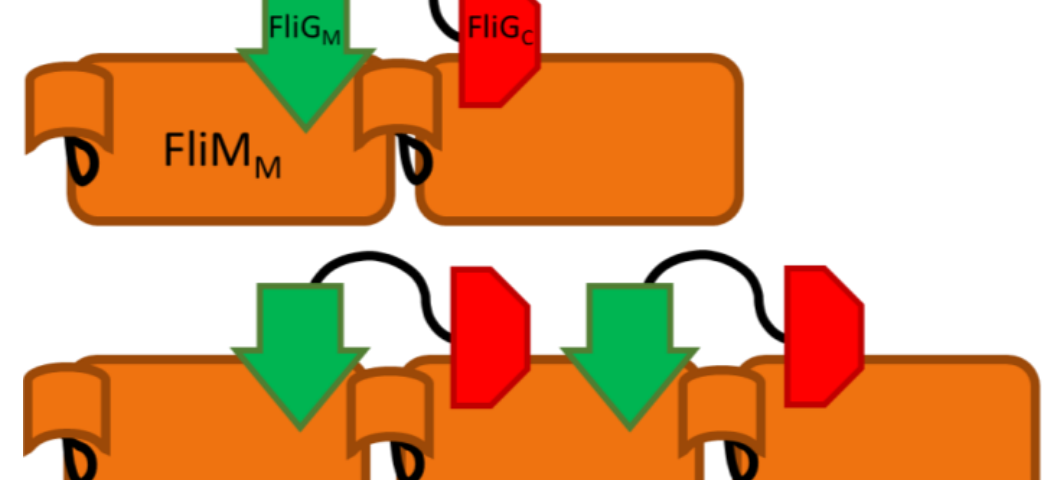
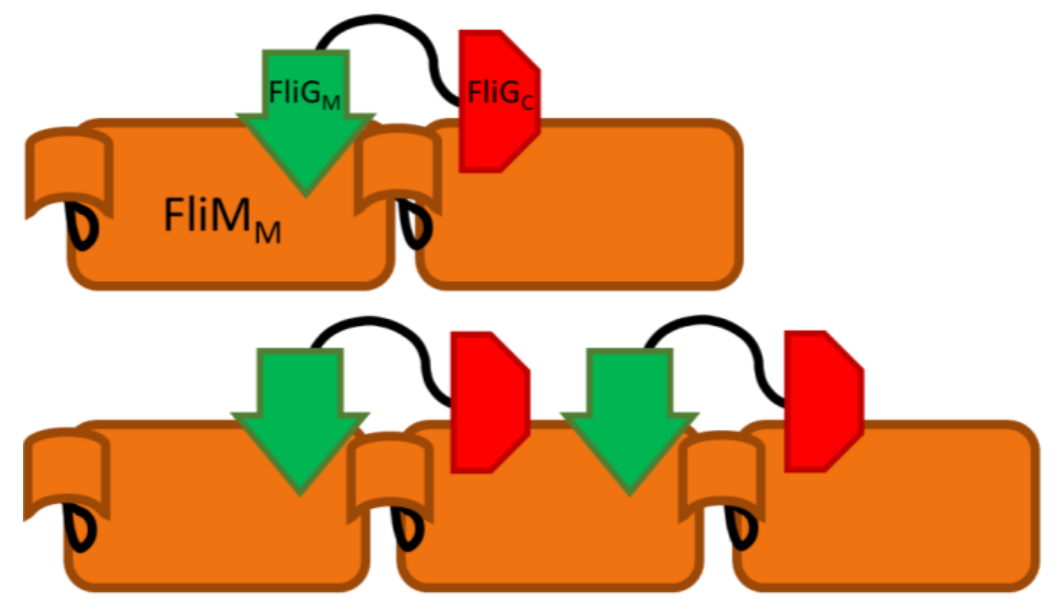
Complex interactions between proteins modulate the rotational direction of bacterial flagella. In particular, the middle and C-terminal domains of FliG (FliGM and FliGC, respectively) bind two different sites on the binding partner FliM, as part of the flagellar motor switch. We extend previous NMR studies of the interactions between FliG domains with FliM via composition-gradient multi-angle static light scattering (CG-MALS) to confirm specific binding, quantify affinities, and identify the stoichiometries of complexes formed.
CG-MALS provided insights into a complicated protein-protein interaction not possible with NMR or traditional techniques. The slow assembly of the multi-domain proteins into higher order complexes uniquely captured by CG-MALS provides direction for future studies and may help determine the mechanism of flagellar motor switch self-assembly.