Characterizing Stable Protein Formulations
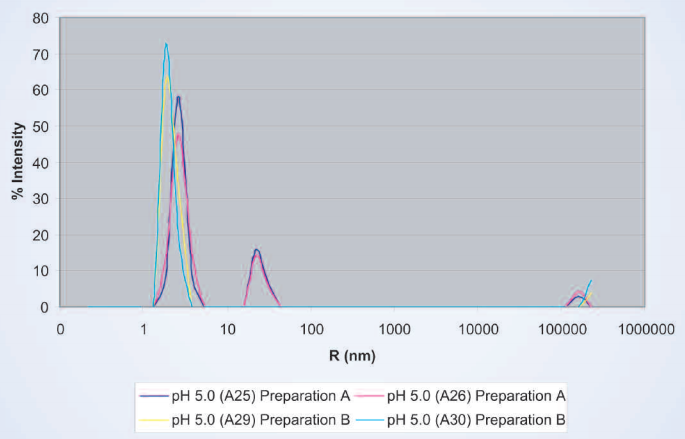
Typical stability problems observed in protein dosage forms are covalent and noncovalent aggregations, deamidation, cleavages, oxidation, and surface denaturation, all of which may cause the protein therapeutic to lose biological activity. Column-free analytical methods such as field-flow fractionation (FFF), analytical ultra-centrifugation (AUC), and dynamic light scattering (DLS) are now increasingly used for aggregation analysis.