LNP formulation, development & characterization
Challenges in LNP Formulation Development
Developing optimal lipid nanoparticles (LNPs) requires them to be safe, effective, and cost-efficient. Achieving this involves exploring numerous LNP formulation and process parameters, coupled with thorough characterization. Researchers must employ high-throughput analysis to evaluate extensive sample sets, alongside detailed analysis to understand why certain formulations are more effective than others.
The LNP formulation process involves three key steps: selecting the appropriate lipid components, incorporating the payload into the lipids, and characterizing the final product. The choice of lipid components and their composition depends on the target tissue, payload, and delivery method. The search for the best lipids and compositions is rapidly evolving to suit various targets and delivery methods, including injection, oral, and inhalation routes. New lipids are continuously being tested and developed to match these delivery methods and target tissues. Testing various lipid formulations for both safety and efficacy can result in creating a vast library of samples. Moreover, because LNP formation is a kinetic process, the method of mixing significantly impacts the final product's characteristics.
Addressing these challenges demands an iterative approach, continually refining and optimizing formulations to meet stringent criteria for successful LNP development.
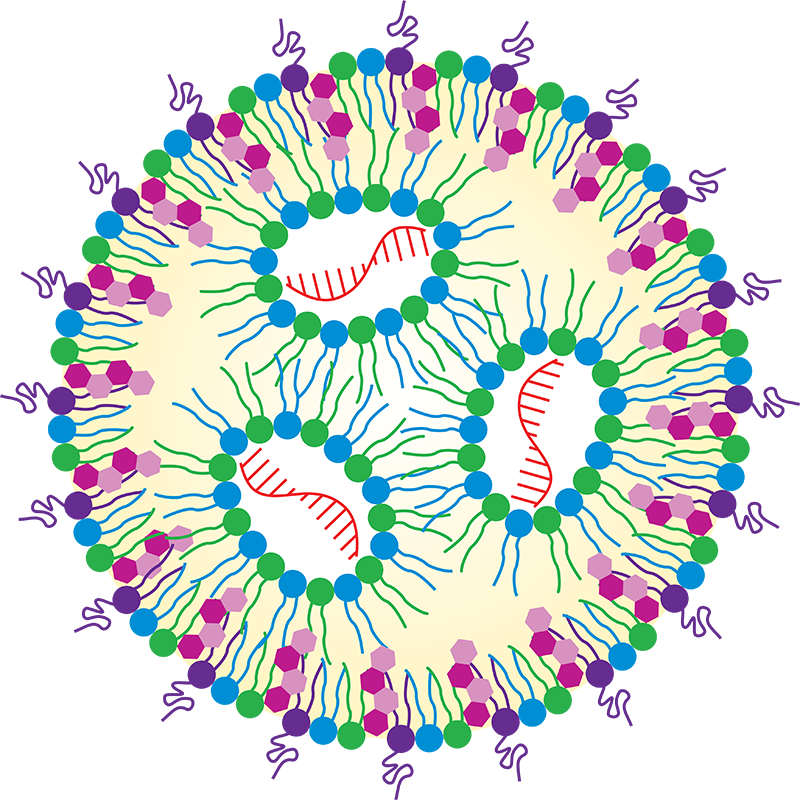
LNP Composition

LNP formulations typically involve a combination of lipids such as phospholipids, cholesterol, ionizable lipids, and PEGylated lipids. Neutral lipids like phospholipids form the LNP's core structure, while ionizable lipids facilitate endosomal escape, enhancing payload delivery efficiency. PEGylated lipids improve LNP stability and circulation time in the bloodstream, and cholesterol maintains structural integrity and regulates the release rate. This mix forms stable nanoparticles capable of encapsulating and safeguarding therapeutic agents such as nucleic acids, proteins, or small molecules. Check out some example composition and molar ratio of commercially available LNP therapeutics[1].
| Patisiran | BNT162b2 | mRNA-1273 | ||||
| Component | Molecule | Molar Ratio | Molecule | Molar Ratio | Molecule | Molar Ratio |
| Ionizable Cationic Lipid | DLin-MC3-DMA | 50% | ALC-0315 | 46.3% | SM-102 | 50% |
| Neutral Phospholipid | 1,2-DSPC | 10% | 1,2-DSPC | 9.4% | 1,2-DSPC | 10% |
| Cholesterol | Cholesterol | 38% | Cholesterol | 42.7% | Cholesterol | 38% |
| PEGylated Lipid | DMG-PEG(2000) | 1.5% | ALC-0159 | 1.6% | DMG-PEG(2000) | 1.5% |
| Cargo | siRNA | mRNA | mRNA | |||
LNP Mixing Methods
LNP formation is a kinetically driven process, so the method of mixing plays a crucial role in determining the final product's characteristics. To achieve maximum efficacy, each LNP formulation needs to be optimized for specific biophysical characteristics such as shape, size, payload density, and surface charge.
At the bench scale, LNP mixing processes can be classified into three categories: manual mixing, mixing with the aid of a liquid handler, and using dedicated microfluidic devices. These methods vary in terms of cost, maintenance requirements, and the precision of the final product.
- Manual Mixing: Methods like pipette mixing involve the least initial investment and excel in making LNPs with minimal sample consumption. However, they produce LNPs with the highest polydispersity.
- Liquid Handlers: Automated preparation with a liquid handler significantly increases throughput. Depending on its flexibility, the same liquid handler may also be used for other parts of the workflow, such as RNA extraction.
- Microfluidic Devices: Various microfluidic mixing models exist with different working volumes, throughput, and automation capabilities. Microfluidic systems are generally recognized for producing the most repeatable process and LNPs with the narrowest size distribution. However, this does not guarantee product success because the optimal size may not have been produced.
The mixing method is not a guarantee to an effective LNPs. Recent studies show that the total delivery efficacy of LNPs generated with microfluidics ranges from 100-fold less to 4-fold more than manually mixed LNPs[2]. Better physical characterization and correlation to functional assay performance is needed to make LNPs with the desired structure.
LNP Production Process
Making LNP production involves several processing steps. First, a LNP formulation stock solution containing the organic lipid components in ethanol is prepared, along with a separate stock of mRNA. Typically, the formulation uses a 3:1 ratio of organic to aqueous solution[3]. These solutions are then mixed using either automated or manual methods. After mixing, the LNP mixture is quickly diluted or buffer-exchanged to reduce ethanol concentration, as lipids are semi-mobile in ethanol and will continue to grow in size, potentially altering encapsulation over time.
The LNPs are then re-concentrated in the final buffer and cleaned up. Size selection can be tailored using a 0.2 µm filter for small-scale production or a column for larger batches. Minimizing sample loss is crucial to increase efficiency as each step after mixing can introduce sample loss due to degradation and aggregation.

LNP Characterization Methods
Throughout the LNP formulation development process, the type of analysis required evolves. During the lipid formulation testing stage, a large LNP library is often created by varying the lipid components and their compositions. High-throughput solutions are essential for efficiently managing the numerous samples. Techniques such as plate-based dynamic light scattering (HT DLS) and the Ribogreen assay are highly effective for initial screenings. For more detailed analysis, zeta potential measurements provide insights into surface charge, while advanced techniques like field-flow fractionation multi-angle light scattering (FFF-MALS) and cryo-electron microscopy (Cryo-EM) offer high-resolution data on size distribution and payload distribution. This detailed information is essential for understanding why some LNPs perform better in functional assays.
A non-exhaustive example of the various LNP characterization methods can be found below.
 |
 |
 |
|||||
| High Throughput DLS | DLS/SLS/ELS | SEC/FFF-MALS | LC-MS | NTA | CryoEM | Fluorescence-based assays |
|
|---|---|---|---|---|---|---|---|
| High throughput | |||||||
| Automatable | |||||||
| mRNA integrity | |||||||
| mRNA identity | |||||||
| Particle size | |||||||
| Size Distribution / Polydispersity | |||||||
| Particle concentration | |||||||
| Zeta potential | |||||||
| Colloidal stability | |||||||
| Payload concentration | |||||||
| mRNA payload distribution | |||||||
| Encapsulation efficiency | |||||||
| Lipid composition | |||||||
| Lipid concentration | |||||||
| Lipid modification |
* Fluorescence based assays includes PCR and RiboGreen Assay
Conclusion
Developing effective LNP formulations is a complex, iterative process requiring a combination of high-throughput screening and detailed analysis. By optimizing lipid components, mixing methods, and characterization techniques, researchers can create LNPs that meet the stringent criteria necessary for successful therapeutic delivery.
Citations
- Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids, C.M. O’Driscoll et al., Drug Discovery Today, 2023
- The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism, K.A. Whitehead et al., Eur J Pharm Biopharm, 2023
- LipidLaunchTM LNP-102 Exploration Kit, Cayman Chemicals, 2024
