I found the perfect mobile phase for my experiments. Now how do I find the right validation standard?

Introduction
Wyatt detectors enable experiments using a broad range of solvents and mobile phases. However, when you switch mobile phases you’ll need to revalidate your system with an appropriate molecular standard.
Why validate with a standard? Validation is different than calibration. Calibration is performed in toluene once or twice a year to determine the MALS calibration constant. Validation uses a standard to set three parameters: normalization, alignment and band broadening.
Normalization
Normalization makes the calibration constant compatible at angles other than 90°. Changing your mobile phase will change the scattering angles requiring re-normalization using a sample that scatters light equally in all directions—which is typically any sample where all components have a radius of 10 nm or less. Passing your samples through 0.02 µm (20 nm diameter) filter is a good way to ensure normalization won’t be influenced by anisotropic scattering.
Alignment
Alignment corrects for the interdelay volume between detectors and band broadening compensates for the peak broadening that occurs as the sample goes through the detectors. For these parameters, the size of the sample does not matter. Instead, a monodisperse sample is required to ensure all peaks can be aligned to each other. The alignment and band broadening corrections from this monodisperse sample can be checked once a year—unless the tubing between the detectors changes these values should remain fairly constant.
Mobile Phases
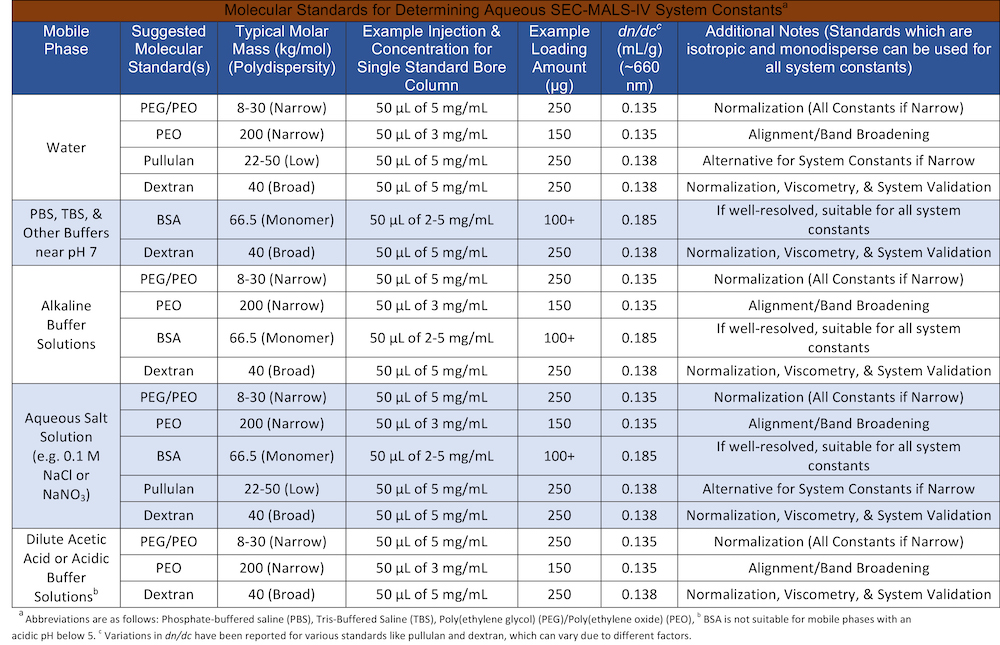
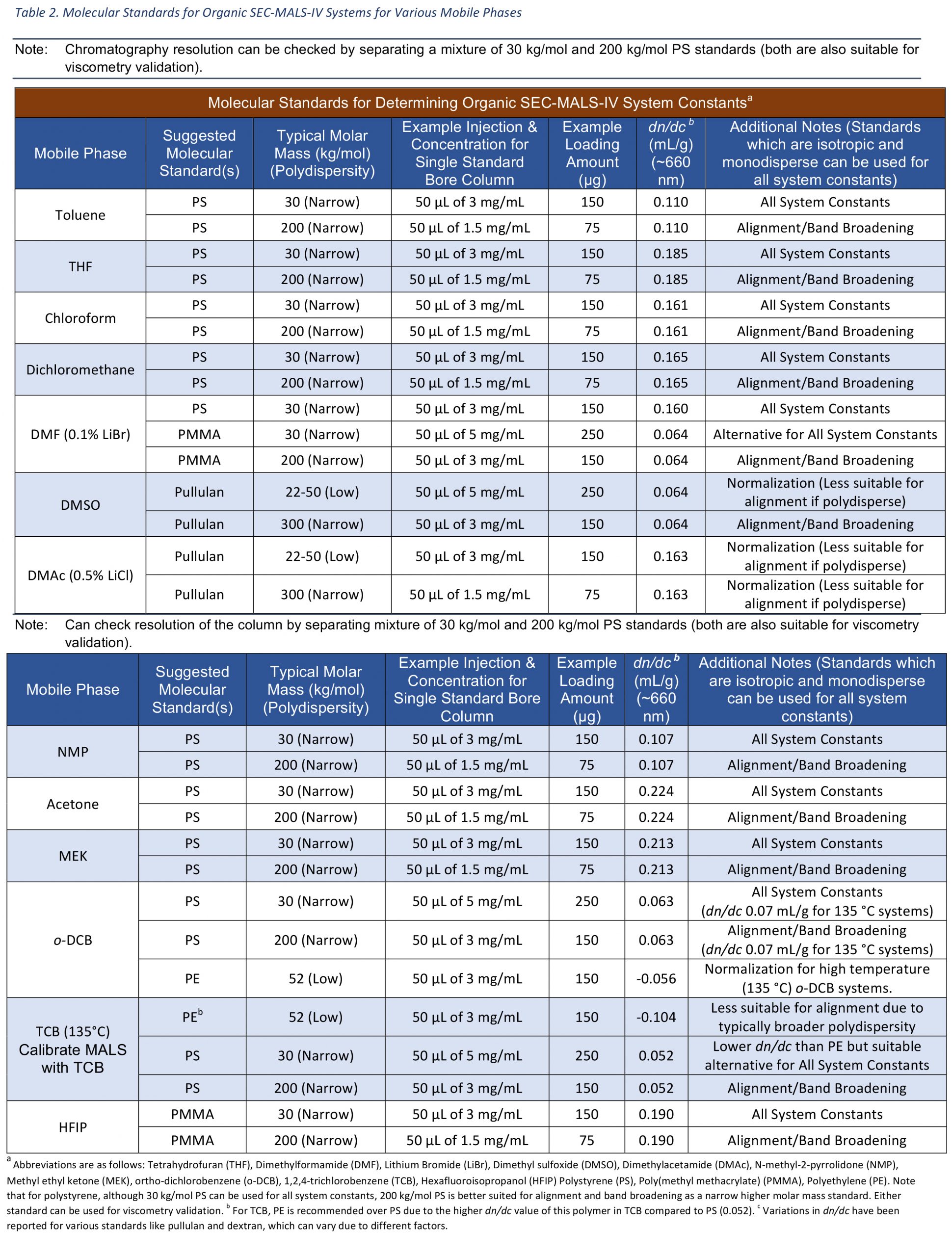
Below are two tables that show some standards that can be used for different mobile phases. The first table details molecular standards for aqueous systems while the second table details molecular standards for organic systems. For molecular standards like BSA, separation of the monomer from dimer can give insight into the effectiveness of your chromatographic separation. For organic systems, mixing polystyrene (e.g. nominal 30 and 200 kg/mol standards) is a good way to ensure your columns are separating well.
Table 1. Molecular Standards for Determining Aqueous SEC-MALS-IV System Constants for Various Mobile Phases.
Note: Chromatography resolution can be checked by ensuring separation of a polymer mixture or BSA monomer, dimer, and higher aggregates.
Conclusion
Do you have a mobile phase that isn’t listed? Not sure what you can use to determine these parameters? Keep in mind that normalization needs only a small (10 nm or less) scatterer. This includes many proteins and polymers with a molar mass less than 100 kDa. So finding any small macromolecule that is soluble in your desired mobile phase can work for normalization—even if it is polydisperse. Additionally, both Alignment and Band Broadening corrections are related to interdelay volumes between detectors. If you can’t find a monodisperse sample for your mobile phase, you can determine these constants in a different mobile phase and use them after you switch back to your desired mobile phase.