AAV – Challenges in Characterization and Bioprocessing

February 5, 2024
AAV Excitement and Barriers
We live in an exciting time as we are able to witness the successful cures of genetic diseases that were once considered untreatable. A critical aspect of these breakthrough gene therapies lies in the delivery of their genetic payload, which is often achieved by viral vectors, especially the adeno-associated virus (AAV). As of December 2023, there were five AAV gene therapies that have been approved by the FDA offering treatments for hemophilia A and B, SMA and retinal dystrophy. Additionally, there are over 450 AAV treatments in various stages of pre-clinical and clinical development.
Even with all this excitement, this area has experienced difficulties with some companies abandoning AAV delivery because of challenges in characterization and manufacturing. The yields of these therapies are poor while the cost of goods is extremely high compared to other commercialized biotherapeutics. When speaking with scientists in this area some have mentioned that only 5-15% of the manufactured drug makes it to the patients due to low yields and high volumes required for release testing.
How does Waters | Wyatt Technology™ address this issue?
AAVs are far more complex than traditional biologics and present new challenges to product and process developers, as well as to their analytical support teams. Wyatt offers a suite of measurement techniques—comprising both traditional and automated analytical tools—to support the characterization and multi-attribute quantification of adeno-associated viruses.
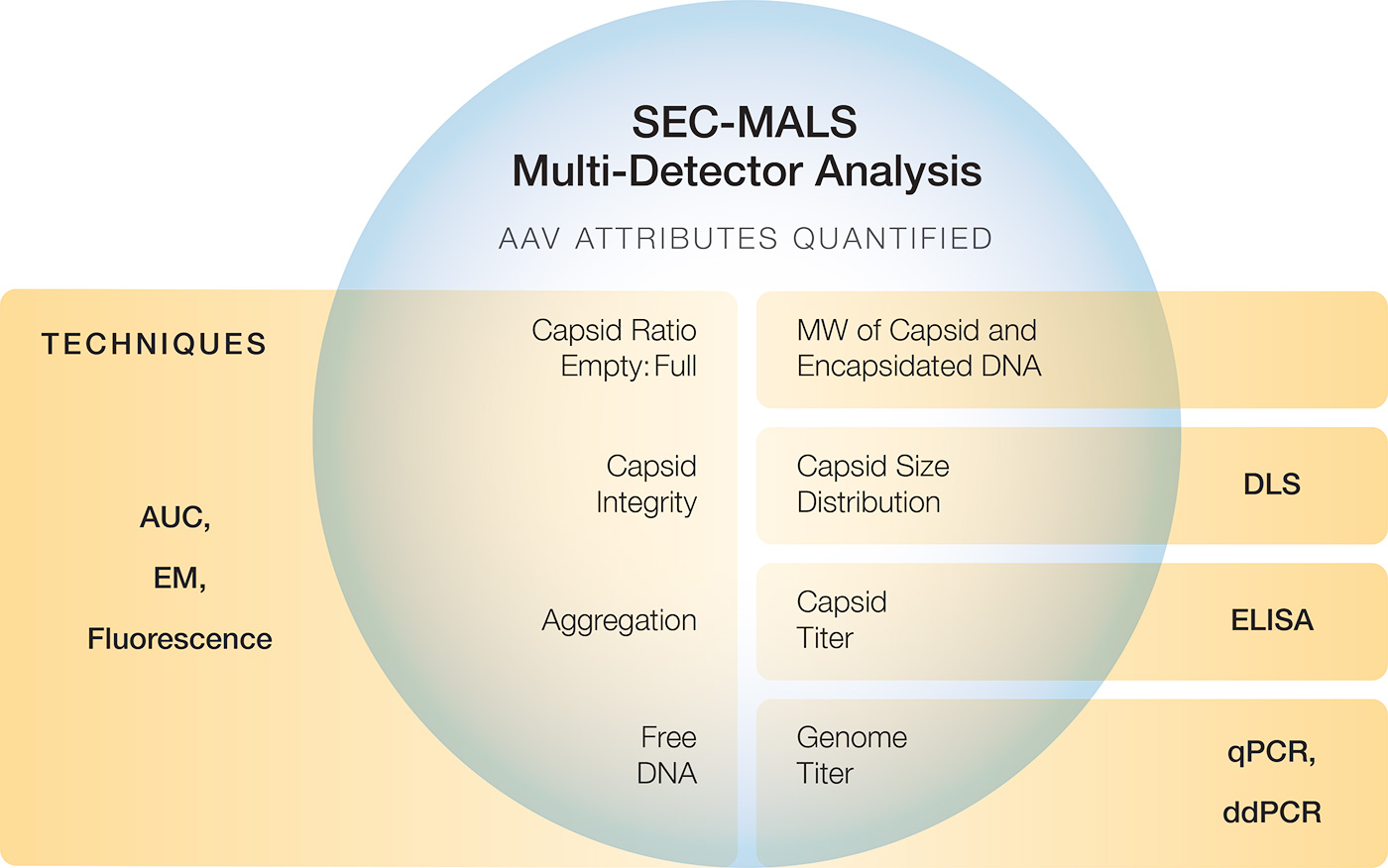
Our solutions for characterizing gene vectors, based on static and dynamic light scattering, quantify up to three critical quality attributes in a single measurement:
- particle concentration
- aggregate content
- thermal stability metrics such as onset of aggregation
For extended characterization, Wyatt instrumentation determines:
- UV extinction coefficients of capsids and encapsidated DNA
- capsid and genome molecular weight
- viral particle size
- molecular weight of impurities such as free DNA
- stability
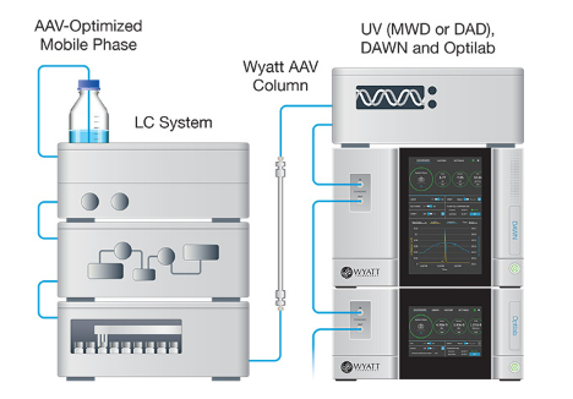
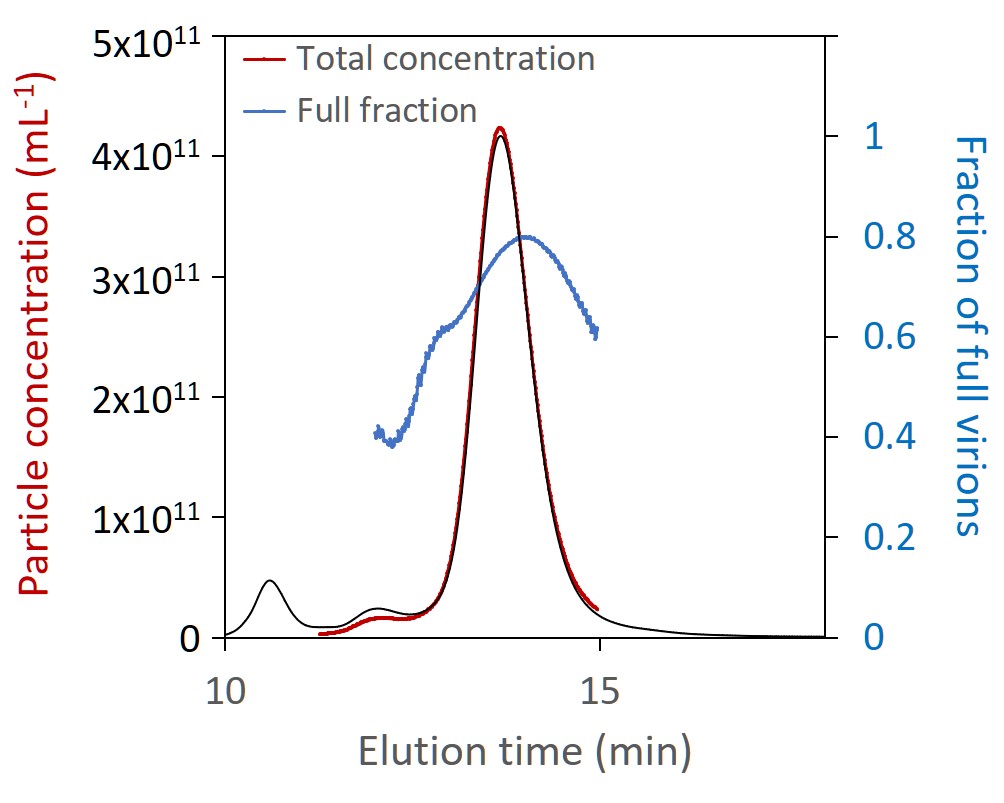
The workhorse method for critical quality attribute (CQA) quantification and extended characterization is SEC-MALS. Coupling size-exclusion chromatography to Wyatt’s industry-standard DAWN™ multi-angle light scattering and Optilab™ refractive index detectors and supported by a comprehensive guide to preparing and validating standard operating procedures, SEC-MALS has become a platform method for automated AAV characterization. Results closely match those of other common techniques, and the limit of sensitivity is just 5 x 1010 capsids/mL. Wyatt’s unique Method Implementation and Training™ offering for SEC-MALS analysis of AAVs ensures that the instruments are utilized to the fullest, from the start.
It is essential to be able to characterize aggregates that are too large for SEC columns, positioning FFF-MALS as the go-to technique for detailed stress and stability studies. Field-flow fractionation separates particles up to 1000 nm in size, and the Eclipse™ FFF system couples to the same detectors as SEC-MALS to provide similar analytics over a larger size range.
Quick, low-volume studies of stability, aggregation and overall capsid titer can be carried out in our DLS instruments:
Dynamic light scattering determines size, size distributions and particle concentration without a separation step and so take just seconds per sample. Formulation and stress screening studies involving dozens or hundreds of samples can be automated in the DynaPro Plate Reader which accepts standard 96, 384 or 1536 well plates.
Beyond the analytical lab, RT-MALS is a process analytical technology for in-line or on-line monitoring of downstream purification and fill-finish processes. The ultraDAWN™ real-time multi-angle light scattering detector measures the same product attributes as SEC-MALS, albeit with less detail since it does not use an associated separation method. In process development, coupling an ultraDAWN to the process enables accelerated development with far fewer offline analyses. In production, RT-MALS supports intelligent process control and can be used to optimize pooling, indicate a process endpoint or flag CQA deviations.
References:
Nóbrega, C. Mendonça, L.; Matos, C. A Handbook of Gene and Cell Therapy. Springer Cham. Springer Nature Switzerland AG 2020. https://doi.org/10.1007/978-3-030-41333-0
Wang, D., Tai, P.W.L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 18, 358–378 (2019). https://doi.org/10.1038/s41573-019-0012-9
Naso, M.F., Tomkowicz, B., Perry, W.L. et al. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31, 317–334 (2017). https://doi.org/10.1007/s40259-017-0234-5
FDA website. “Approved Cell and Gene Therapy Products”. Accessed Jan 24, 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
Loyd, I. Pharma R&D Annual Review 2023. Citeline Clinical. April 2023. Downloaded Jan 24, 2024.
Want to learn more?
Check out our web page on AAV characterization and quantitation, which has links to relevant publications, app notes and webinars. Or request information by clicking the button below.



