Colloidal Stability and Zeta Potential
The successful development of therapies, personal care products, and certain foods requires precise understanding of the colloidal properties of their components. Colloidal stability refers to the ability of a system to resist changes in particle size. If the system is balanced in terms of repulsive and attractive forces, and the particles are not changing in size, then it is considered stable. This property is fundamental in determining whether a product’s developability and suitability for a particular application. There isn’t a direct way to measure colloidal stability; instead, it is interpreted through a few different values. Zeta potential, the measure of surface charge, is one of the primary measurements.

What is the zeta potential?
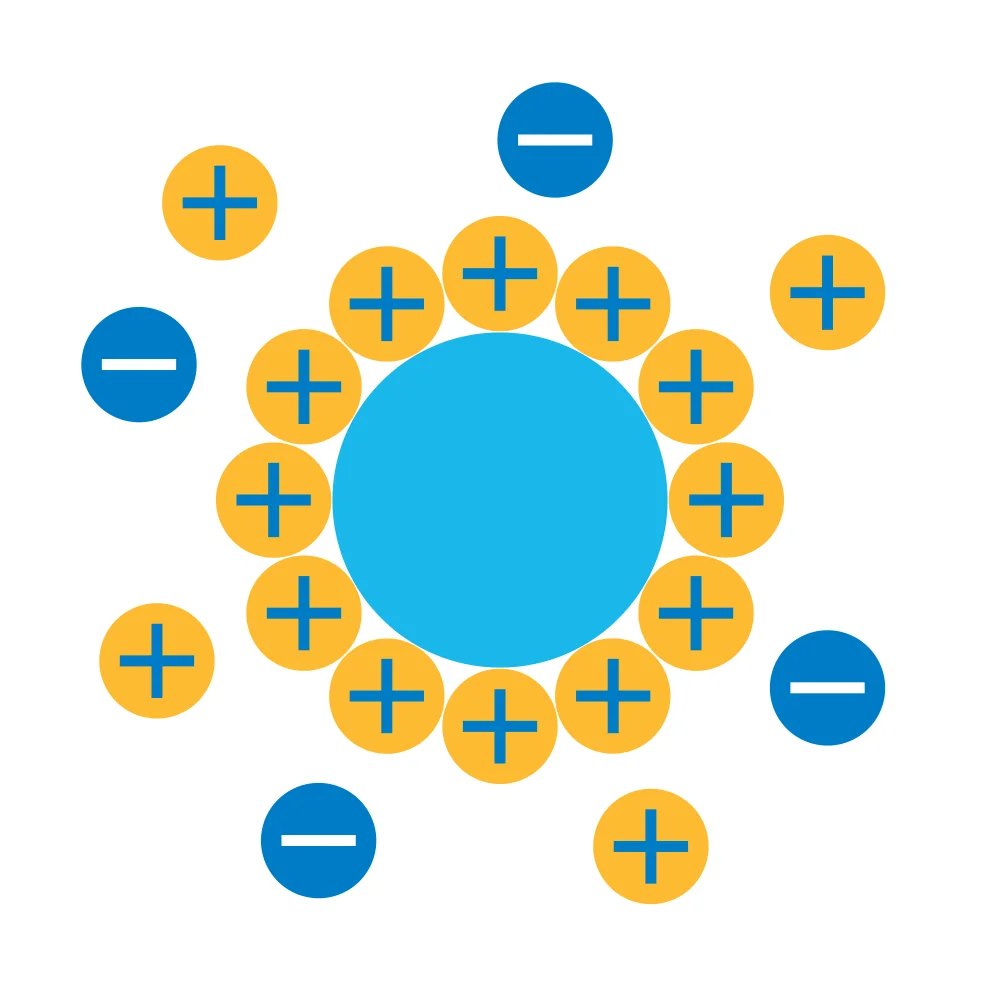
Zeta potential is the electric potential arising from a particle’s surface charge and attached counterions. The determination of this property provides detailed insight into mechanisms such as dispersion, aggregation, and flocculation. It is also useful in determining the pKa of polymers or the pI of a protein. It can be used to answer questions such as: "Will this lipid nanoparticle have favorable pharmacokinetics?" or "How does a molecule’s property change when a surfactant is added?" This knowledge can be used to improve the development of biologics and nanoparticles.
Why is the zeta potential important?
The zeta potential value indicates the potential stability of a colloidal system. If all particles in a suspension have a high positive or negative zeta potential, they repel each other. If, on the other hand, the zeta potential is low, there is no force to prevent the particles from colliding and flocculating. The boundary between stable and unstable suspensions is usually between -30 mV to +30 mV.
How do you measure zeta potential?
Zeta potential is typically measured by observing a particle’s movement in an electric field. The particle’s movement’s speed during this process is a measure of the electrophoretic mobility. The mobility, combined with the hydrodynamic radius (Rh), allows for accurate calculation of the zeta potential.
How is zeta potential influenced?
The zeta potential can be influenced by numerous factors, including the pH, ionic strength, and the addition of excipients or additives. The buffer plays a significant role; changing the pH of the solution impacts the ionizable groups either through protonation or deprotonation and the ionic strength of the buffer can mask charged groups. Surfactants, like detergents such as SDS or polysorbates will also modify the surface and the observed charge.
Zeta potential applications
Suspensions like cosmetics also benefit from zeta potential measurements during development. Skin care products are typically formulated as an emulsion and understanding the zeta potential helps to predict its behavior and colloidal stability. This ability to measure and predict stability via zeta potential reduces development time, as product stability can be determined early in the process.
Lipid nanoparticles (LNPs)
In the context of lipid nanoparticles (LNPs), colloidal stability or instability can affect drug efficacy through particle aggregation. Zeta potential helps gauge these interactions by measuring the forces between charged particles and can determine whether a system is stable or prone to aggregation. The drug delivery properties of an LNP are influenced by its zeta potential with positively charged species that easily penetrates a cells membrane and negatively charged species having a longer circulation time in the bloodstream. Researchers also modify the LNPs surface to control the release of its therapeutic payload. The charge of an LNP is also important for biocompatibility particles with a high or low zeta potential induce a negative immune response. Understanding and controlling zeta potential is critical for LNP therapies and impacts their stability and all aspects of its pharmacokinetics.
Measuring zeta potential with instruments from Waters | Wyatt
Our portfolio includes various DLS analyzers with which you can quickly and easily determine the zeta potential as well as other values.
To calculate the zeta potential of a particle, you need its electrophoretic mobility and hydrodynamic radius. The former can be determined using electrophoretic light scattering (ELS), the latter using dynamic light scattering (DLS). Our DynaPro™ ZetaStar™ ELS/DLS/SLS instrument provides simultaneous measurements of dynamic light scattering (DLS) and electrophoretic light scattering (ELS), offering insights into particle size, polydispersity, and zeta potential in one efficient process. Furthermore, simultaneous measure of DLS/SLS provides size, polydispersity and particle concentration, molar mass and turbidity. Also, because the instrument’s flow cell can be pressurized, dissolved gases that can confound signals are avoided, thereby allowing measurements under physiological conditions.
If you want to determine the zeta potential of each component in a mixture, the Eclipse™ Field Flow Fractionation (FFF) system with the Mobility EAF4 module is the right choice. It works with electrical asymmetric field flow fractionation and can also determine zeta potential distributions of multi- and polydisperse samples.
Either way, zeta potential measurements are in reach with light scattering measurements. We recommend the measurement early and often in development and deployment as a quick QC check with the final product.
References:
- Mo, S., et al. "Increasing entropy for colloidal stabilization" Scientific Reports 6 (2016)
- Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol. 697 (2011):63.
- Xu, Letao, et al. "Lipid nanoparticles for drug delivery" Advanced NanoBiomed Research 2.2 (2022): 2100109.
- Seo Y, et al. “Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications” Pharmaceutics. 15(3) (2023): 772.
- Hong, S. et al. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 12 (2020): 604. https://doi.org/10.3390/pharmaceutics12070604
- Kamble, S. et al. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 7(1) (2022). Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements (wiley.com)
Featured Instrument:
DynaPro™ ZetaStar™

Fast & automated DLS/SLS and electrophoretic light scattering (ELS) instrument.
