Quality standards for the life sciences: PQS
Daniel Some, Ph.D.
Governmental agencies such as the FDA and EP enforce rigorous guidelines to ensure the quality of research, development and production of food substances, pharmaceuticals and other materials on which modern society relies. We have high confidence that the life-enhancing drug we purchase tomorrow will be the same as the one we purchased yesterday, with similar therapeutic effect and side effects. All of society benefits from these quality guarantees.
Value vs. waste
Science is one of the greatest endeavors of the modern age. Probing and understanding the natural world from quarks to quasars and everything in between, science not only satisfies our curiosity and expands our conscience, but enriches our material lives with technology that eliminates drudgery and disease. Rightly, society pours vast amounts of money and human capital into the scientific enterprise.
But how much of that capital is wasted? I am not referring to the pursuit of apparently useless or uninteresting research, for usually we cannot anticipate the value of directions of research, or even assign value to them. Nor am I referring to duplication of effort, which is often a good thing despite every researchers1 desire to produce novel, ground-breaking science. Instead, I refer to good-faith, well-supported lines of research resulting in efforts wasted due to lack of reproducibility, which is a direct outcome of insufficient quality control of source materials and protocols.
Lack of reproducibility has been at the center of recent discussion in the life sciences community. The journal Nature devoted a special issue and web page to this topic: see https://www.nature.com/news/reproducibility-1.17552. Concerns about rigor and reproducibility have come up in many areas of the life sciences and the community has been tackling it on many fronts, from ethics to education. The establishment of well-defined guidelines for rigor, even if implemented on a voluntary basis, would constitute a major step in the right direction to minimize wasted monetary and human capital in this field.
Measure twice, publish once
In this spirit, the Protein Production and Purification Partnership in Europe (P4EU) and and the Association of Resources for Biophysical Research in Europe (ARBRE MOBIEU) have recognized the need to establish guidelines for the verification and characterization of recombinant proteins utilized in research:
There is increasing awareness in the scientific community about the lack of reproducibility and reliability of results published with purified proteins. While protein production is highly regulated and controlled in the pharmaceutical industry by the authorities, there are up to date no guidelines or standards in place in the academic research to guarantee the quality of proteins included in scientific experiments. There is an urgent need to define guidelines for the scientists but also for editors and reviewers of scientific journals and funding agencies for their review processes.
(P4EU web site, https://p4eu.org/protein-quality-standard-pqs, accessed 1/25/2018))
These organizations have assembled a team of experts to develop a Minimum Protein Quality Standard aimed at improving the reliability and reproducibility of protein-based research.
The PQS draft is published on the web at https://p4eu.org/protein-quality-standard-pqs and https://arbre-mobieu.eu/guidelines-on-protein-quality-control/. It establishes clear and straightforward recommendations for the minimum set of biophysical assays that should be performed on every such protein to ensure its suitability for use as in experiments, and calls for the results to be publish in every peer-reviewed paper describing such experiments. Some examples of the assays listed include purity testing by SDS-PAGE or capillary electrophoresis (CE), aggregation testing by size-exclusion chromatography with multi-angle light scattering (SEC-MALS) and/or dynamic light scattering (DLS), and conformation/folding analysis by circular dichroism (CD). The proposed standard includes recommendations for minimum additional information on the production and handling of the proteins to be included in publications, such as concentration (by UV absorbance at 280 nm or otherwise) and a record of storage conditions.
Wyatt Technology recognizes and supports the establishment of this PQS, and is pleased to note that that its products such as the DAWN, miniDAWN and µDAWN MALS detectors and the DynaPro NanoStar DLS detector are essential components in protein characterization laboratories across the globe, used by many members of the team that produced the PQS. Light scattering covers many areas of biophysical characterization, including molar mass, size, charge, interactions, conformation and conjugation, and is applicable from peptides to proteins to exosomes and beyond. More information about the extensive types of protein characterization that are made possible by Wyatt instruments is available at www.wyatt.com/Proteins and www.wyatt.com/Library.
Adopt a standard today!
While more careful scientists will often go beyond the PQS guidelines in terms of quality control assays and reporting, many biologists are probably unaware of the need for this degree of quality control. I believe that the simple existence and publication of the PQS will gradually lead to its adoption and implementation, for the overall benefit of the scientific community and the public at large. The adoption process could be greatly sped up if journal editors and reviewers took note and began recommending or requiring the inclusion of the PQS data in publications. Perhaps even more relevant would be the adoption of the PQS by funding agencies such as the NIH and NSF, in order to minimize the waste of public funds and to increase confidence in government management of research.
References:
- Quality assessment and optimization of purified protein samples: why and how? Raynal et al. Microbial Cell Factories 13:180 (2014)
- The Trip Adviser guide to the protein science world: a proposal to improve the awareness concerning the quality of recombinant proteins. Mario Lebendiker, Tsafi Danieli and Ario de Marco, BMC Res Notes. 7:585 (2014 Sep 1).
- Reagent validation: an underestimated issue in laboratory practice. Ario de Marco. J. Mol. Recognit. 23, 136 (2010)
- Recombinant protein quality evaluation: proposal for a minimal information standard. Buckle, A.M et al. Standards in Genomic Sciences 5, 195-197 (2011)
- Daviter T, Fronzes R: Protein Sample Characterization. In Protein-Ligand Interactions: Methods and Applications. Edited by Williams MA, Daviter T. Totowa, NJ: Humana Press; 35–62 Methods in Molecular Biology, vol 1008 (2013).
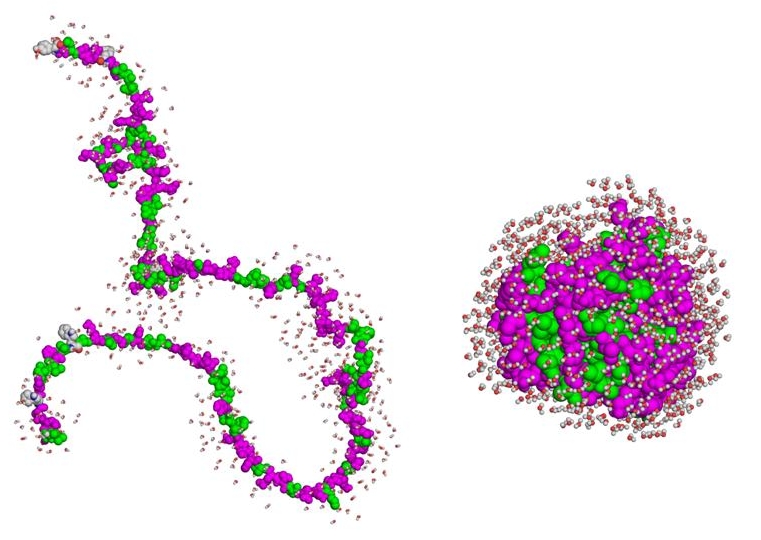
1. Denatured proteins may remain soluble yet be incompetent to interact
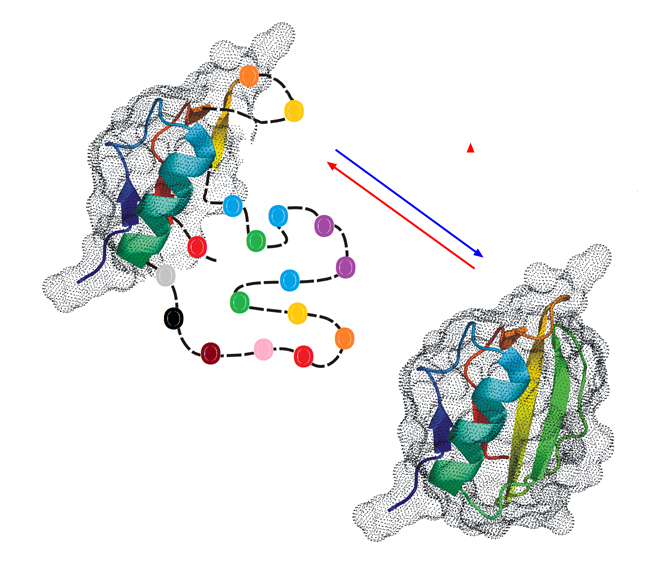
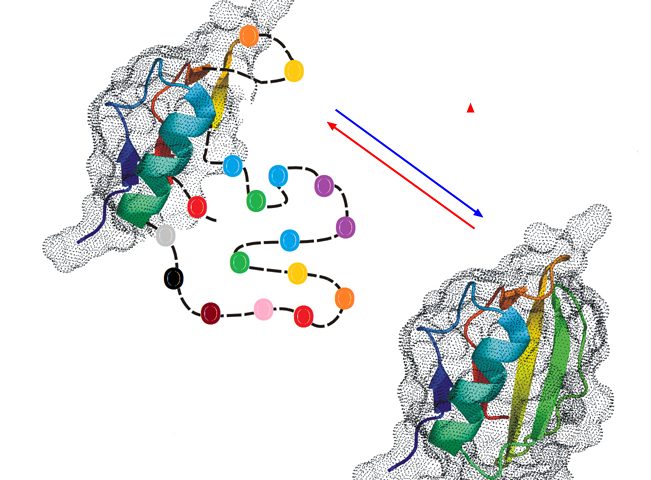
2. Partial unfolding or inherent disorder may interfere with biological activity
3. Protein impurities may produce false binding signals
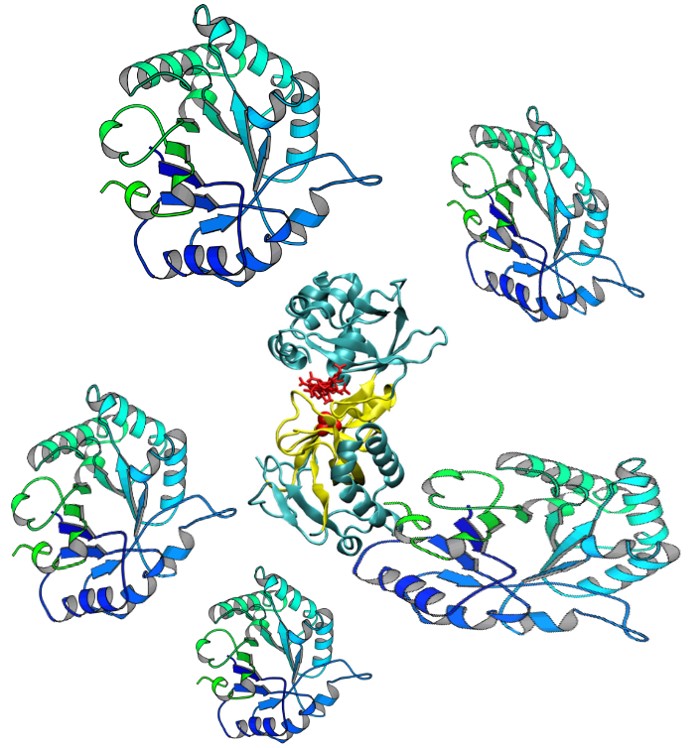
4. Establishing the native oligomeric state is essential to understanding activity
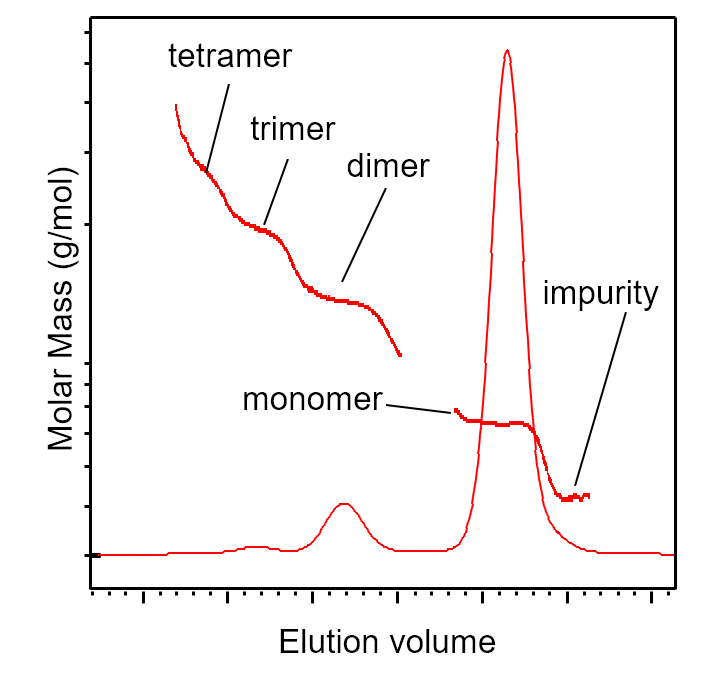
5. SEC-MALS identification of aggregates and impurities
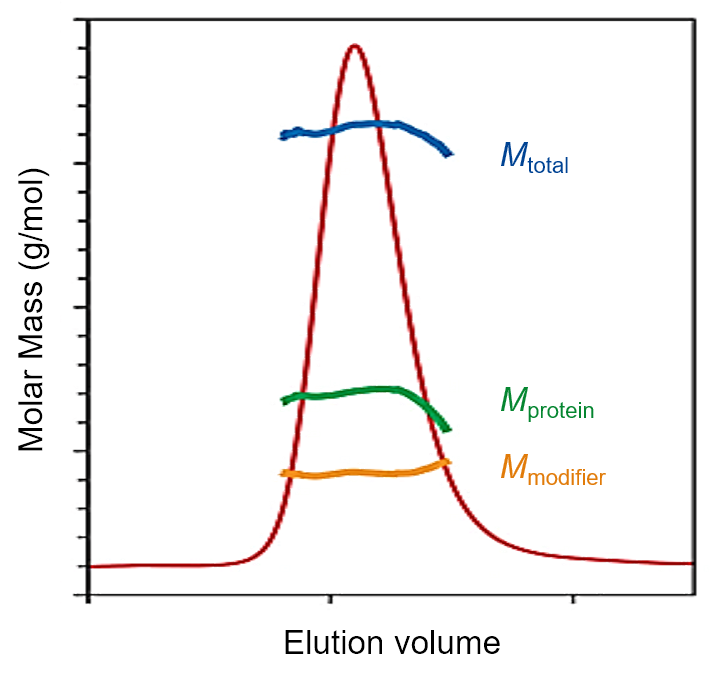
6. SEC-MALS determination of glycosylation or membrane protein loading of lipids
