What resources does Wyatt offer for to quantify LNP quality attributes with SEC/FFF-MALS?

Introduction
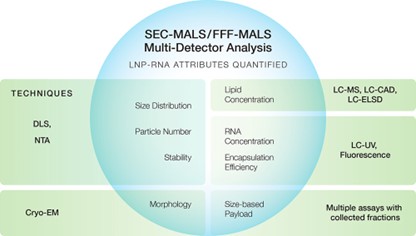
The LNP Analysis module in the ASTRA™ software enables high-resolution multi-attribute quantification (MAQ) of lipid nanoparticle (LNP)-formulated nucleic acid and protein therapeutics by FFF-MALS or SEC-MALS. FFF-MALS is our preferred solution, especially for medium and large-sized LNPs, due to its wide size range and tunable separation capabilities. This analysis is featured in our application note AN2615: Multi-attribute quantification of LNP-mRNA therapeutics by FFF-MALS and DLS.
In this article, we present several key resources that can help accelerate your LNP characterization by providing direct measurements of multiple LNP quality attributes from single experiments, without the need for calibration curves or laborious multi-step assays.
LNP Guidance Manual
Our tailor-made LNP Guidance Manual is accessible directly from the Help menu in the ASTRA software. This comprehensive guide includes step-by-step protocols for data acquisition and analysis of LNPs by FFF-MALS or SEC-MALS. We describe method settings, data acquisition, and analysis in detail to support development of a Standard Operating Procedure, and providing expert guidance on data interpretation, analysis, and troubleshooting.
The guide is clearly laid out and fully searchable, and you can view and print sections as desired. Like all our products, we continuously update and improve the LNP Guidance Manual with expert advice on experimental design, data analysis, and troubleshooting, delivering the most up-to-date documentation through software updates to ASTRA.
Dedicated LNP Analysis Methods
The LNP Analysis module expands upon other conjugate analyses within ASTRA. Create and save optimized LNP methods using ASTRA’s Method Builder, customized for your specific instrument parameters. We’ve also included new System methods specifically designed for LNP sample analysis, for additional processing using functions like Apply Method or One-to-Many. If you are using FFF-MALS to analyze your LNP samples, VISION™ software offers additional features, such as starter FFF methods optimized for LNPs and the ability to simulate and optimize separations from the ground up or from existing experimental results with VISION DESIGN™.
Accurately Interpret UV Absorption and Scattering
LNPs are typically large enough to absorb and scatter light at commonly used UV wavelengths. Consequently, even a sample of empty LNPs, devoid of nucleic acid or protein payload and made with non-UV-absorbing lipids, will give a UV signal due to UV scattering. The LNP Analysis module enables the user to correct for such UV scattering, thereby unlocking accurate payload concentration measurements from UV absorbance detectors. Save customized Empty LNP profiles to your product-specific methods to ensure accurate and robust analysis every time.
In-depth, Size, Polydispersity, and Payload Distribution
With the LNP Analysis module you can perform size-based payload analysis directly on intact LNPs, without having to extract the drug substance from the particles. Quantify multiple attributes from a single experiment, including the LNP size distribution, particle concentration, morphology, lipid concentration, and the mRNA concentration and number either averaged across the whole sample or as a function of LNP size.
DLS Analysis for Rapid Screening
Dynamic light scattering (DLS) offers a quick complement to high-resolution FFF/SEC-MALS measurements. The high-throughput, low volume measurements of the DynaPro™ Plate Reader provide fast estimates of size and particle concentration that are easy to implement at any stage of the LNP product and process development process. The DynaPro™ ZetaStar™ can also deliver automatable batch measurements of LNP zeta potential at high precision. To learn more about our DLS instruments and solutions for LNP analysis, read our application note AN6601: Measuring size, concentration, and zeta potential of LNPs with the DynaPro ZetaStar.
Conclusion
Wyatt provides a full suite of instruments and methods for complete quantitation and characterization of your LNP products, including FFF-MALS, SEC-MALS, and DLS solutions. To learn more about our automatable and 21 CFR Part 11 compliant solutions for LNP analysis, please visit the Solutions for Lipid Nanoparticle (LNP) Characterization in Product & Process Development section of our website.
Do you have a question? Contact our experts here in Customer Support. We’re happy to help! Call +1 (805) 681-9009 option 4.