RT-MALS: Real-time product attributes, from lab to plant

Updated March 10, 2023
Wyatt’s analytical instruments, based on multi-angle static light scattering (MALS) and dynamic light scattering (DLS), have played essential roles in the development of biotherapeutics for over three decades. Early applications specialized in determining absolute molar mass, size, charge, conformation, conjugation, aggregation and interactions of therapeutic proteins such as IgG(1); polysaccharides like hyaluronic acid(2); antigenic glycoproteins including viral surface motifs (3); and viruses or virus-like particles as vaccines(4). More recently the DAWN™ MALS instrument and DynaPro™ DLS detectors have been enhanced with the latest cutting-edge capabilities to enable characterization of the titer and genomic content of viral and non-viral gene vectors such as adeno-associated virus(5) and RNA-bearing lipid nanoparticles(6). Common to all of these applications of light scattering is their place in the scheme of biologic development and bioprocess: primarily in the analytical and formulation lab, with a few specific instances in quality control.
Given the importance of MALS and DLS instruments in product development, it makes sense to ask how they might benefit process development and as process analytical technologies (PAT). In fact, MALS and DLS are often used for off-line analytics during process development. Depending on the properties of the specific product, especially (but not always) size range, fractions collected during downstream processing (DSP) may be analyzed by SEC-MALS, FFF-MALS and/or batch DLS in automated plate format to assess size or molar mass, polydispersity, aggregate and impurity content, titer and payload. The value of such analyses notwithstanding, off-line measurements of product attributes cannot provide the level of immediate feedback that would constitute a game-changing productivity boost for biomanufacturers.
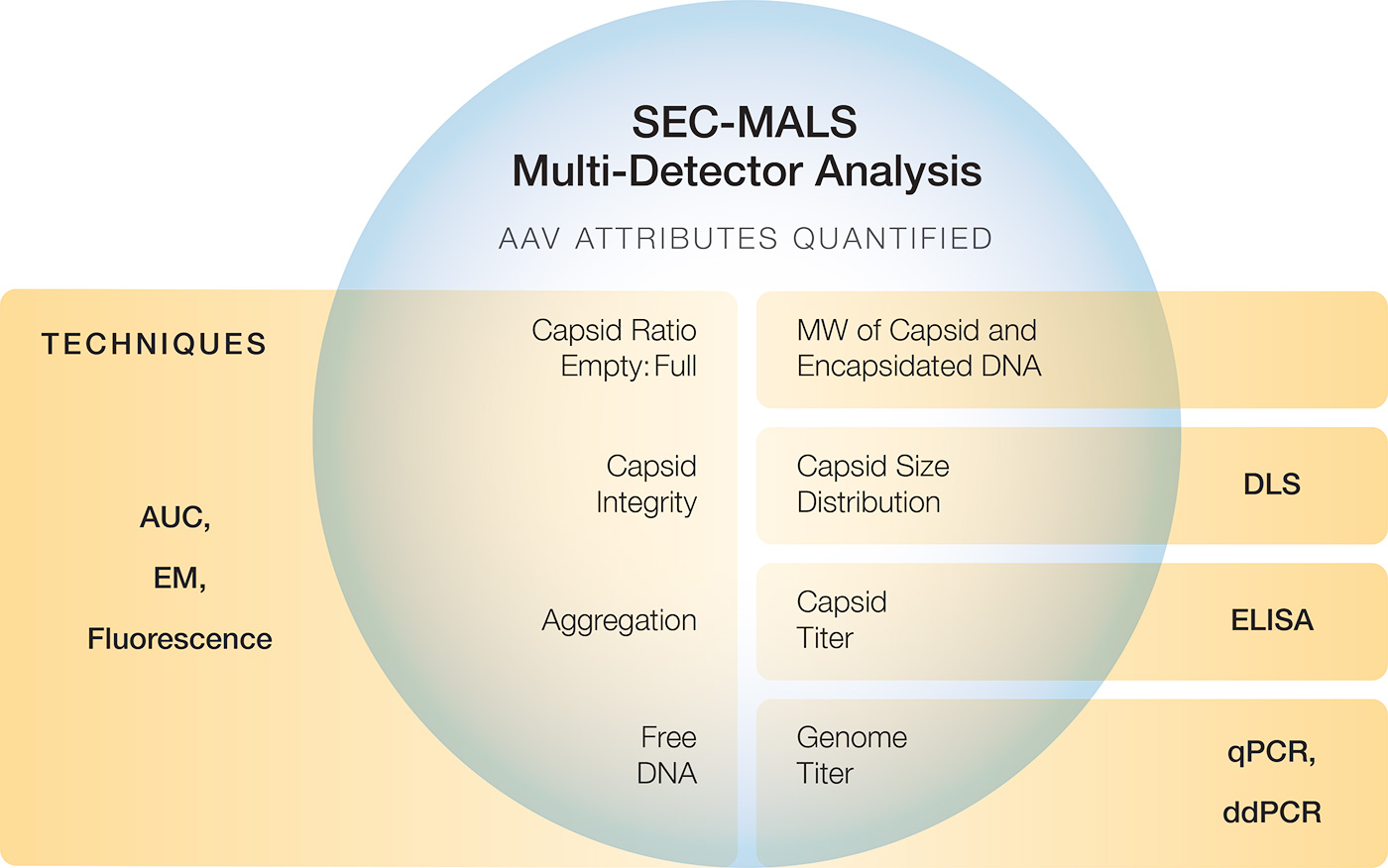
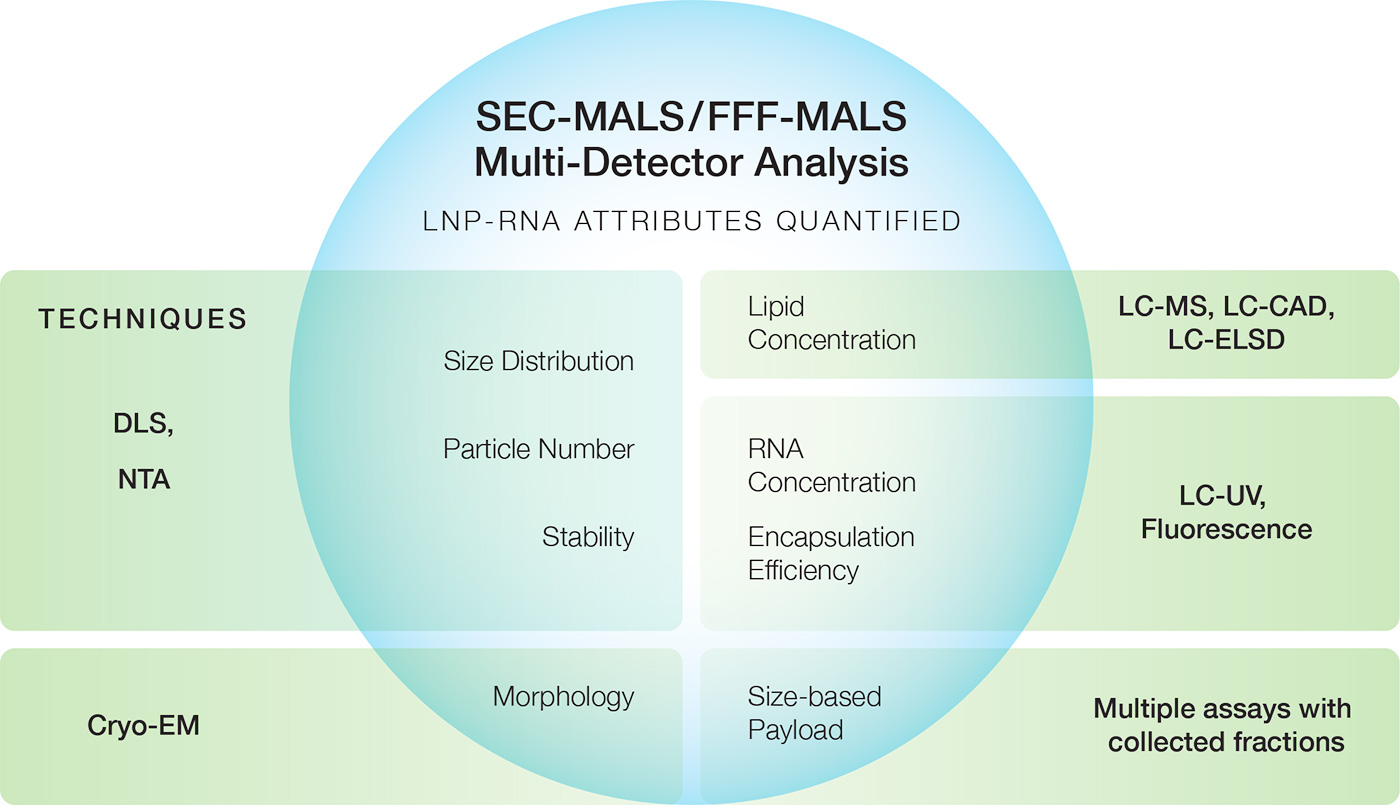
Click to enlarge. Advanced analytical applications of MALS include quantifying multiple key attributes of AAV (top graphic) or LNP-RNA (bottom graphic) in a single, short, reagent-free, automated run, replacing a multitude of individual methods that may require reagents as well as significant time and sample preparation efforts.
PAT and light scattering
PAT is commonly implemented to great effect in many chemical manufacturing environments, but the challenges of bioprocess, including regulatory aspects, have presented significant obstacles to its acceptance in biologics production. Those barriers are starting to come down: increasingly, the FDA is encouraging sponsors to improve yield and quality by adopting PAT, and at the same time there is growing recognition of the potential benefits of PAT on the part of the bioprocessing industry. These benefits include:
- Monitoring and controlling the process in real time
- Streamlining processing, improving yield
- Stopping a bad process early to minimize wasted time, material and resources
- Effectively delivering consistent, quality product
- Advancing to the next stage with confidence
- Transferring successfully across scales and sites
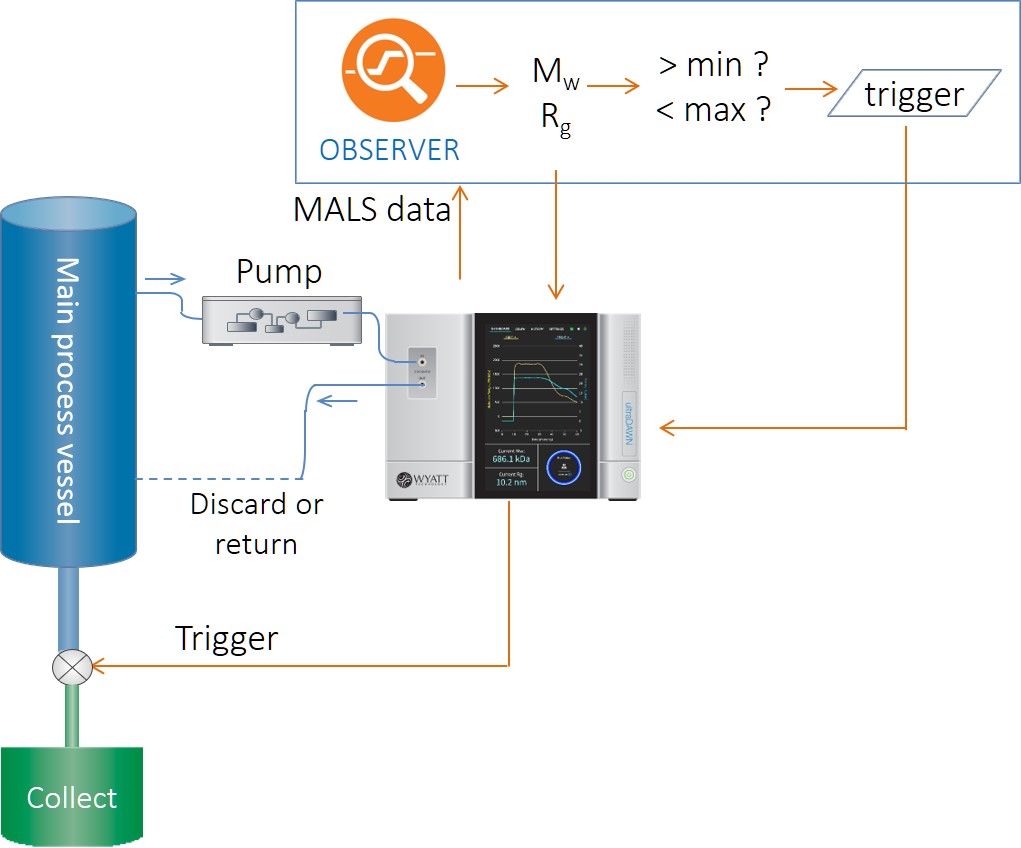
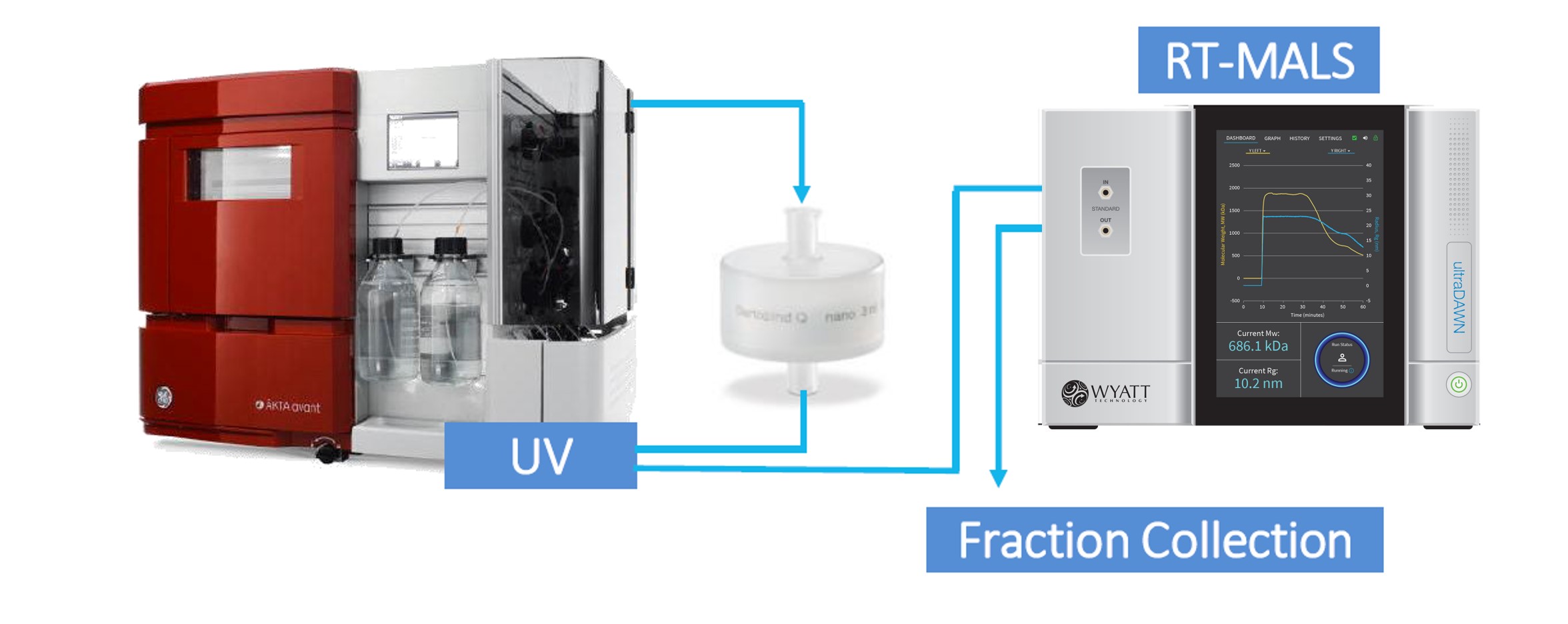
Recognizing the potential of light scattering-based PAT to make a substantial contribution to DSP productivity, Wyatt Technology™ released its first real-time MALS (RT-MALS) products in November of 2019. These consist of the ultraDAWN™ RT-MALS instrument accompanied by OBSERVER™ real-time software. Unlike standard PAT, which monitors process parameters and attributes, RT-MALS sets a new paradigm: it measures, in real or near-real time, actual product attributes. Currently, those attributes are weight-average molar mass, z-average size, and/or particle concentration (physical titer).
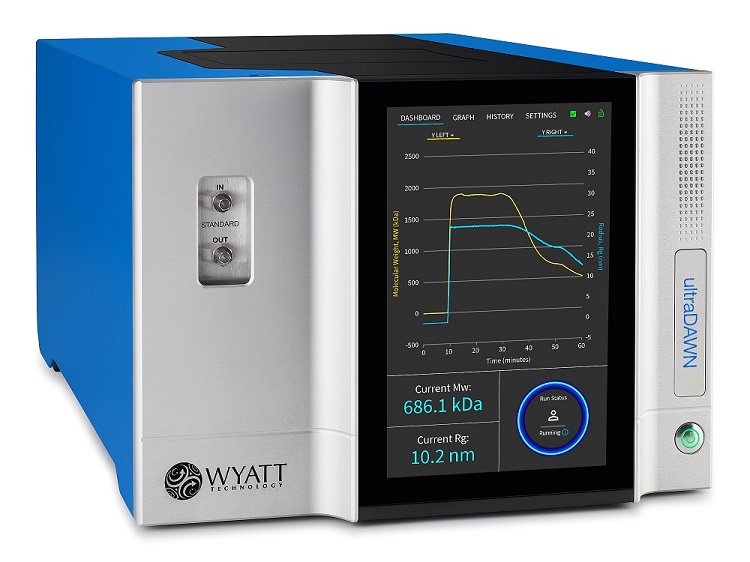
The ultraDAWN measures molar mass, size and particle concentration in real time in order to monitor product attributes inline or online with downstream processes.
Monitor the product, not the process
The prevalent paradigm in biologic manufacturing is ‘the process is the product’. This means that if you understand and have good control over all aspects of the process, you can assure consistent product quality. Hence, standard PAT tools monitor process conditions and indirect product attributes. For example, in a protein purification process, a process attribute (elution volume) or indirect product attribute (UV absorption) may be monitored to decide when to cut a pool in order to minimize aggregate content, even though these parameters are not direct indicators of aggregate content. This approach to PAT may rely on process modelling, entailing a major investment in acquiring the process knowledge needed to build up a reliable model. It also presents an impediment to process improvements which may result in different process parameters, even though the end product is identical or better.
RT-MALS, on the other hand, addresses relevant product attributes head-on. In the previous example, the weight-average molar mass of a protein solution will change in a very specific, predictable and well-understood manner when aggregates begin eluting. Unlike elution volume or UV, variations in the process feedstock or column aging will not cause errors in the pooling cutoff when it is tied to molar mass.
In another application, purification of a virus may require identifying impurities such as free proteins or nucleic acids. UV absorption does not differentiate well between an intact virus and free macromolecules. With RT-MALS, the large size of the virus is exploited to discriminate between it and impurities. In both cases, reliance on process parameters or indirect product attributes necessitates large safety margins resulting in low yield; directly monitoring relevant product attributes with RT-MALS enables smaller safety margins, correspondingly higher yield and enhanced process flexibility – the ability to improve the process without undue burden in re-validation.
Reducing time to market
RT-MALS bridges off-line analytics with real-time response. Whereas off-line measurements might take minutes to hours per fraction (not including sampling time, transit time and lab queues), in-line RT-MALS is essentially instantaneous, providing measurements up to five times per second. For some applications, RT-MALS may be configured on-line, automatically sampling the process vessel or flow path with a pump; in this case, depending on the specifics, it provides data at the same rate as in-line monitoring, but with delay times relative to the process from several seconds up to a few minutes.
In process development and scale-up, RT-MALS means rapid turn-around on the analytics. Instead of sending fractions off to the analytical lab and waiting days or weeks to learn how each tweak to process conditions impacted the production profile, the results are immediate. More conditions may be tested in less time, for faster optimization – and that means that a product can be commercialized and marketed in much less time than usual.
RT-MALS is not a complete substitute for detailed off-line characterization; the tradeoff is time versus detail. An off-line SEC-MALS run provides complete molar mass distributions, while RT-MALS only provides a weight average. On the other hand, SEC-MALS generally runs for 30 minutes or more and requires sampling, transfer to the analytical lab and at least some sample modification, while RT-MALS takes less than a second and is performed in situ. In the end, process development can be sped up greatly with RT-MALS, minimizing—but not completely eliminating—off-line analytics.
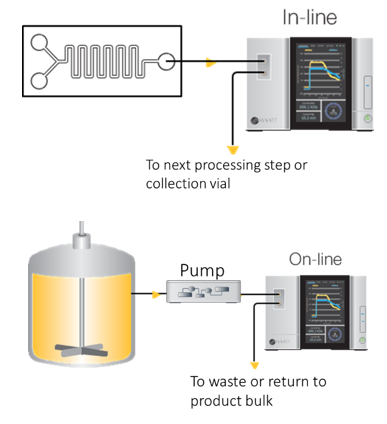
The ultraDAWN may be configured to sample and measure either in-line or on-line, depending on the type of process.
New capabilities and applications
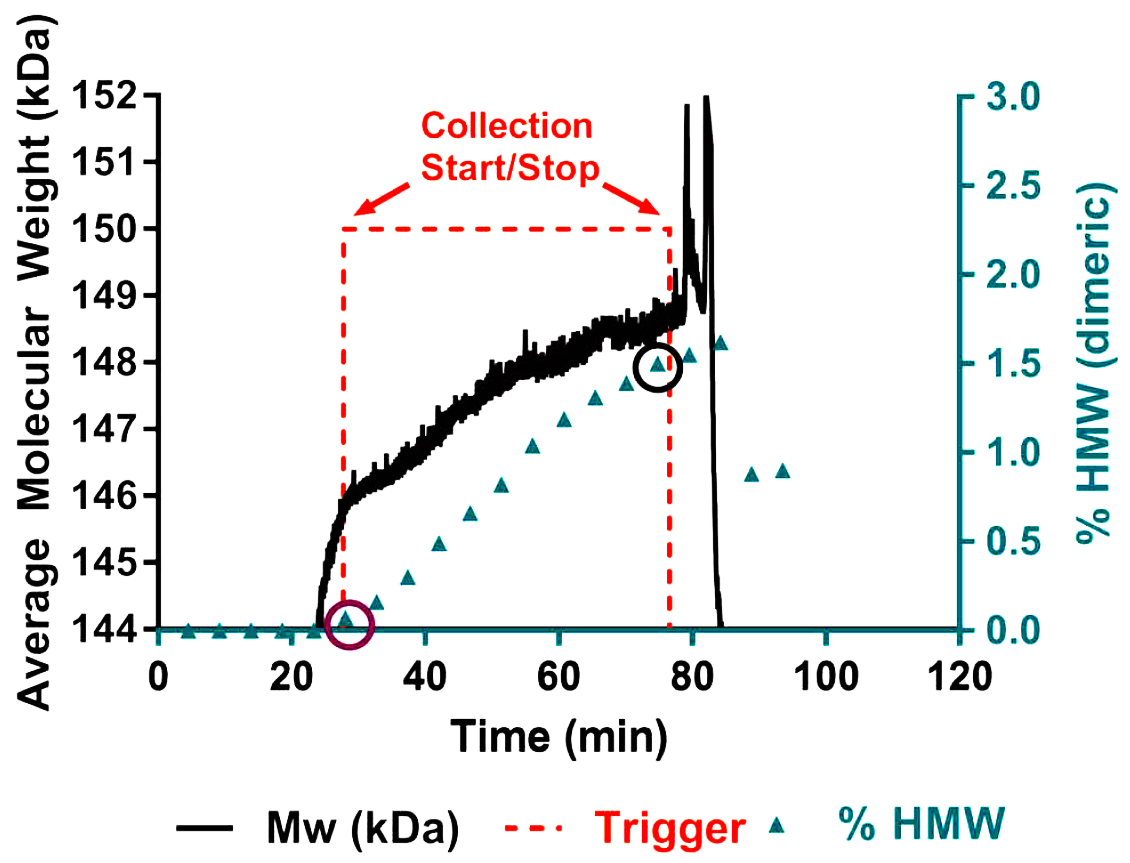
The first publication of RT-MALS for protein purification, by Patel et al. (7), described a prototype in-line RT-MALS system for a flow-through hydrophobic-interaction chromatography (HIC) process. Fortuitously, in flow-through mode the protein concentration and buffer composition are essentially constant during HIC elution, eliminating variability in MALS analysis that may arise from protein-protein interactions that depend on concentration and buffer. The authors monitored aggregate content via changes in molar mass, determined by combining MALS and UV data. The limit of detection was found to be less than 0.25% dimer, enabling precise, real-time pooling decisions that would potentially result in acceptable aggregate content with maximum yield, adding considerable value to each product lot.
OBSERVER 1.0: Online Macromolecule WF
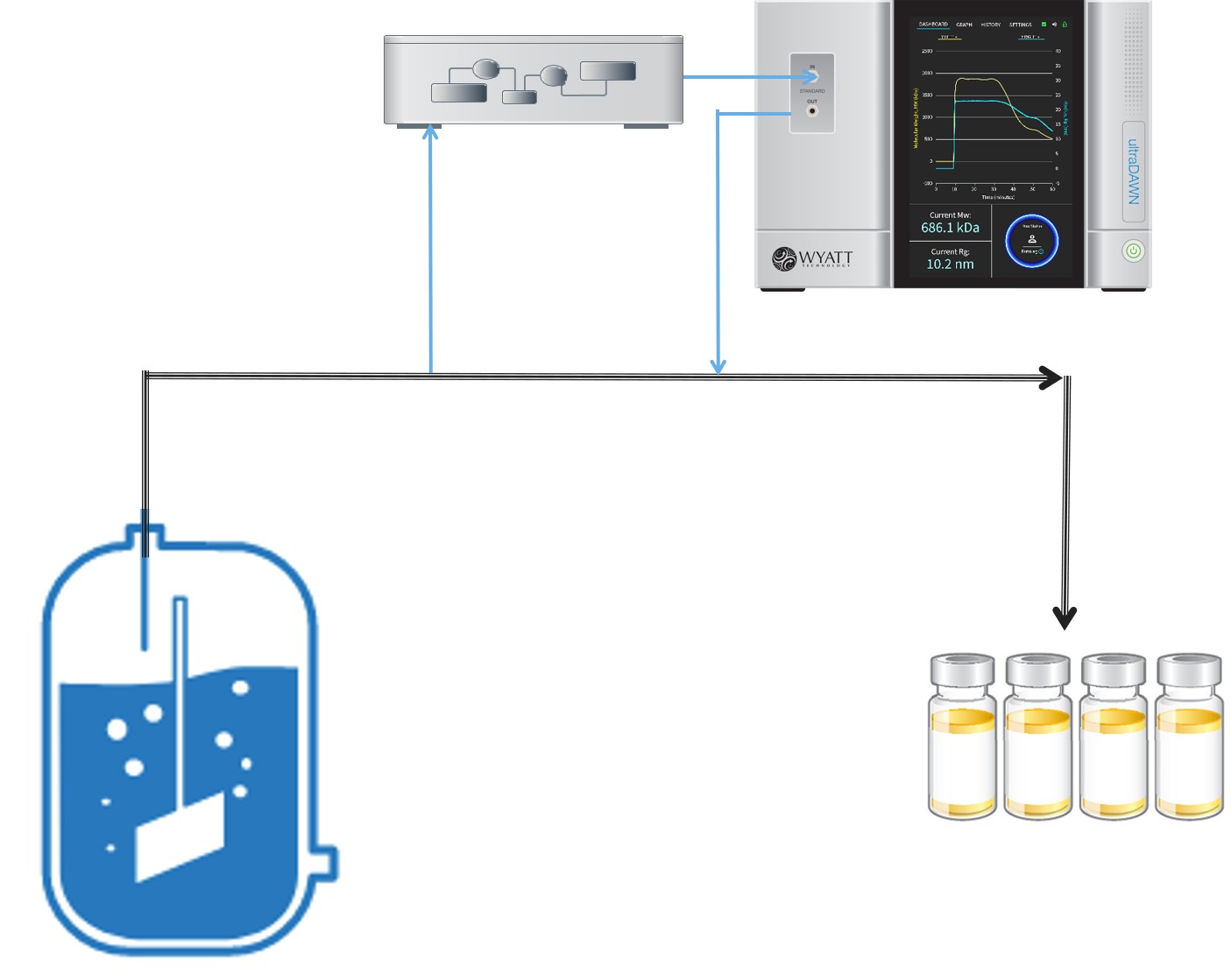
The inital commercial release of ultraDAWN and OBSERVER 1.0 was directed to similar applications, where 1) product concentration and buffer conditions do not change, and 2) the desired product attributes involve molar mass (Mw) and/or size (Rg). Besides continuous-flow protein purification, these include polymerization, depolymerization (e.g. the depolymerization of polysaccharides for vaccines) or the conjugation of proteins and polysaccharides in a reaction vessel.
The polymer reactions inherently require on-line sampling in order to bring sample to the pump, so OBSERVER 1.0 only included an online workflow for molar mass and size reporting: the Online Macromolecule workflow. A quaternary pump, controlled by OBSERVER, is used to aspirate sample from the reactor and dilute with buffer in order to achieve optimal measurement conditions in the ultraDAWN. OBSERVER provides continuous analysis of Mw and Rg, and generates triggers upon entering or exiting a pre-determined range of one of these attributes. An example of RT-MALS application to polysaccharide depolymerization is provided in AN8005: RT-MALS end-point determination of a polysaccharide depolymerization process.
Click to enlarge image. RT-MALS provides immediate indication of increasing aggregate breakthrough in continuous-flow HIC purification. See Patel et al. (7)
OBSERVER’s Online Macromolecule workflow controls a pump to either sample a reactor vessel or a draw a slipstream from a flowing process, and determines average molar mass and size.
OBSERVER 1.1: Online Nanoparticle WF
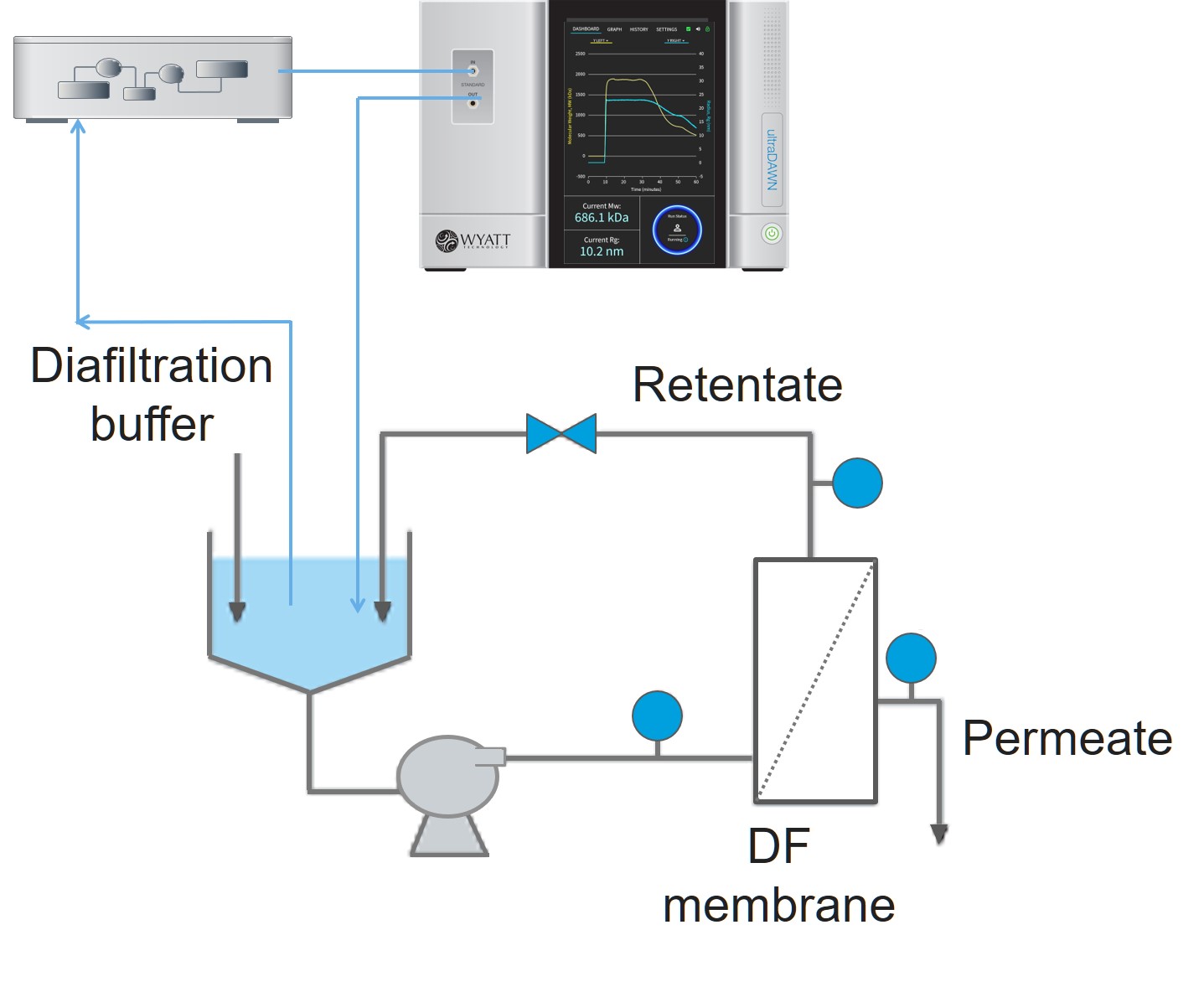
In response to the COVID-19 pandemic and the emergence of novel vaccines based on viral vectors and nanoparticles, OBSERVER 1.1 implemented on-line nanoparticle monitoring. Unlike the Online Macromolecule workflow, OBSERVER 1.1’s Online Nanoparticle workflow calculates particle size and concentration. It is not restricted to constant-concentration scenarios, and because these measurements are not particularly susceptible to changes in solvent composition, it does not require a constant buffer. This makes the Online Nanoparticle workflow suitable for a variety of applications related to nanoparticles, such as:
- viral-vector purification, ultrafiltration / diafiltration and fill-and-finish (especially larger viruses such as adenovirus or lentivirus)
- lipid nanoparticle or liposome formulation development, production and homogenization.
OBSERVER 1.3: Inline Nanoparticle & UV
OBSERVER 1.2 introduced many GUI and behavioral improvements to RT-MALS, but the next key feature appeared in OBSERVER 1.3, the Inline Nanoparticle workflow. This workflow supports applications such as lab-scale chromatographic purification of viral vectors or LNP-RNA formulation and production.
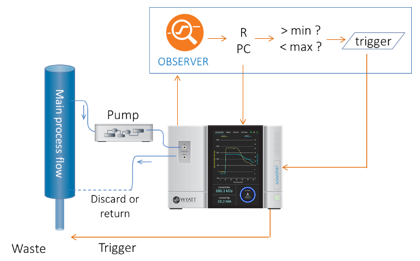
Whereas in online workflows, OBSERVER fully controls sampling and flow to the ultraDAWN, during inline workflows the flow is set by an external system such as an FPLC or a microfluidic LNP formulation device. In these scenarios, OBSERVER must synchronize with the external system. Synchronization is carried out through the simple exchange of digital pulses and analog signals. Example instructions for Cytiva’s UNICORN software to synchronize with OBSERVER are included in the OBSERVER User’s Guide.
In addition to synchronization with the external system, the Inline Nanoparticle workflow exports an analog voltage that is proportional to one of the calculated attributes—size or particle concentration. Hence the real-time data may be imported to the external system for recording, display and even pooling decisions made by its own software, e.g. UNICORN.
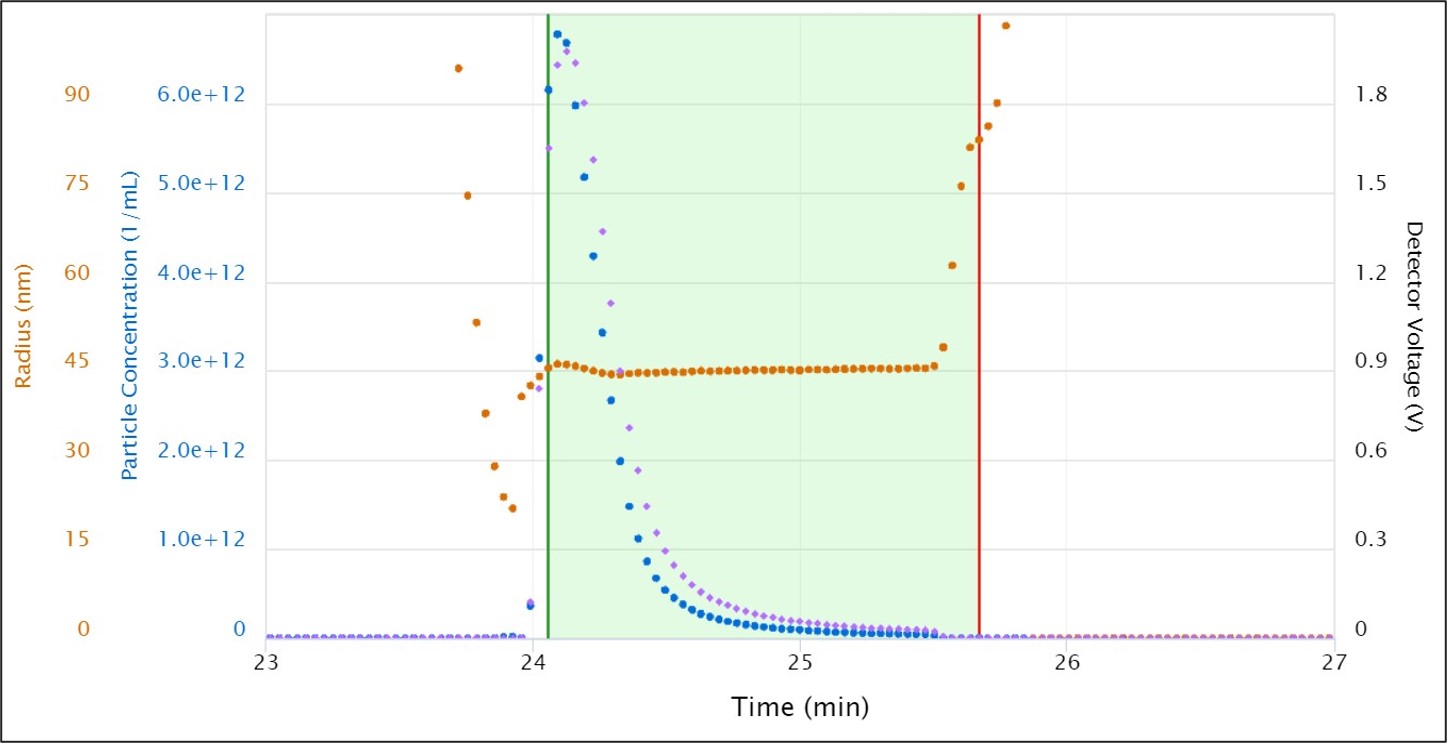
RT-MALS has been demonstrated for in-line monitoring of the elution of an adenovirus-based vaccine product from a lab-scale preparative anion-exchange column.: The study was presented by Clara Pérez Peinado, Janssen Infectious Diseases and Vaccines, at the 2021 International Symposium on the Separation of Proteins, Peptides and Polynucleotides, with example data shown here to the right. Particle radius was used as the criteria for pooling, and viral titer calculated for the total pool. As seen in the figure, instantaneous concentrations above 6 x 1012 virions/mL were measured. Size and final pool titer were in excellent agreement with expected values and off-line analytical methods.
Another new feature in OBSERVER 1.3 is the ability to couple ultraDAWN to a UV absorption detector, in order to determine the molar mass of UV-active molecules such as proteins or nucleic acids, without the constraint of constant concentration. The UV concentration signal is imported as an analog voltage, and was initially available for the Online Macromolecule workflow.
OBSERVER 1.4: Inline Macromolecule WF
The Inline Macromolecule workflow, released with OBSERVER 1.4 in March 2022, takes full advantage of coupling to a UV detector in order to monitor the molar mass of proteins or nucleic acids, with an emphasis on in-line aggregate monitoring such as is described in the Patel mAb article.(7) Intended for protein and nucleic acid purification using lab-scale FPLC and other process equipment, it synchronizes with an AKTA FPLC or similar by exchanging digital and analog signals between UNICORN and OBSERVER via the I/O Box, similarly to the Inline Nanoparticle workflow. The Inline Macromolecule workflow exports real-time analog data for Mw or Rg, as well as a trigger set by criteria based on Mw or Rg.
In-line molar mass analysis is generally limited to conditions where intermolecular interactions are either negligible (concentration in the MALS flow cell is below roughly 1-2 mg/mL and ionic strength > 50 mM) or constant (buffer conditions do not change, such as in flow-through polishing or size-exclusion purification). In the latter case, the virial coefficients A2 and A3 describing the solute-solute interactions of the product in the process buffer should be measured offline and entered into OBSERVER.
For bind-and-elute chromatography with both varying buffer conditions and high eluting protein concentration, an on-line method is preferred. On-line operation sup-ports dilution with a strong buffer of moderate or high ionic strength, reducing both interactions and variations in interactions that arise from variations in buffer pH and conductivity.
OBSERVER 1.4 added another important feature, the ability to print reports detailing the RT-MALS data collected and product attributes calculated during the run, as well as the various settings and parameters that were used in OBSERVER.
OBSERVER 1.5: Inline AAV WF and OPC-UA
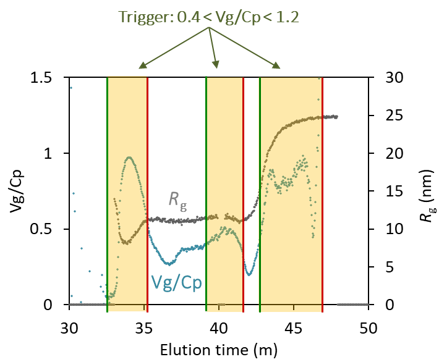
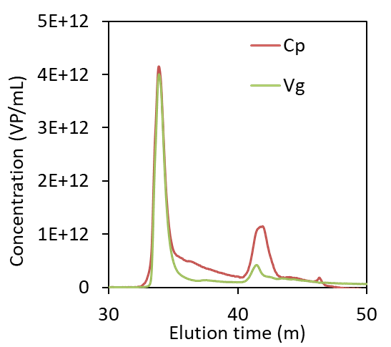
The latest release of OBSERVER, version 1.5 from March 2023, supports PAT in downstream processing of small gene vectors like AAV. The Inline AAV workflow calculates several quality attributes: the full:total capsid ratio Vg/Cp, full and empty capsid concentrations, capsid and average encapsidated genome molar masses and particle size Rg. These attributes are determined by combining MALS with UV260 and UV280 absorption measurements.
- Vg/Cp and full capsid titer are used in process control, to start and stop the collection of genome-enriched capsids during elution from the anion-exchange column. They are also used to optimize column loading and wash steps.
- Empty and full capsid titers are integrated over the collected peak region to determine total resulting titers as well as pool-average Vg/Cp.
- Capsid molar mass and Rg indicate the presence of aggregates and other impurities.
Any one of the measured values can be exported to the process system (e.g. FPLC) as analog values for display, recording and process control. Using RT-MALS, AAV process development scientists obtain pertinent data immediately, in contrast with the delay of days or weeks incurred when fractions must be sent off to the analytical lab. RT-MALS facilitates rapid process optimization and faster time-to-market, followed by tighter process control, higher yields and consistent quality during production.
With OBSERVER 1.5, another new capability of OBSERVER was released: OPC-UA support.
OPC-UA is a communication protocol commonly used in manufacturing environments. It is the most common method of integrating PAT sensors with general PAT software. The ‘OBSERVER OPC’ version of OBSERVER provides the same graphical user interface as standard OBSERVER, but can also become an OPC-UA server, wherein complete control of OBSERVER runs—including data transfer—is performed by an OPC-UA client.
When connected to an OPC-UA client, OBSERVER’s GUI is locked for manual intervention. Hence, if the client provides all the necessary functionality for 21 CFR part 11 compliance such as a secure database, electronic audits, access control, etc., the RT-MALS system can be fully GMP compliant. While Wyatt does not write OPC-UA clients on behalf of RT-MALS customers, the purchase of an OPC-UA license includes one year of unlimited support to help customers interface with OBSERVER, and continuing sup-port is offered with a Gold or Platinum Service Plan for the ultraDAWN. The addition of full OPC-UA support is critical for extending the horizon of RT-MALS to include GMP facilities.
OBSERVER’s Inline Nanoparticle workflow synchronizes with an external system such as an AKTA avant with UNICORN chromatography software to optimize pooling based on size. OBSERVER also provides immediate determination of virion titer in the collected pool.
Real-time measurement of adenovirus elution from an anion-exchange purification column, showing particle size and concentration, and the region identified for pooling based on size. Data courtesy Clara Pérez Peinado, Janssen Infectious Diseases and Vaccines.
Real-time measurement of AAV elution during ion-exchange purification. Top: full:total capsid ratio Vg/Cp and particle size Rg (top). Bottom: capsid particle and viral genome concentrations. The shaded regions were identified for pooling based on Vg/Cp. Increasing radius at ~ 43 minutes is indicative of aggregates.
Outlook
The current ultraDAWN offering fulfills a sizeable subset of DSP PAT applications in the realm of biologics, gene vectors and nanomedicines, primarily in-line for lab-scale process development, and on-line, for sampling of in-place processes (reactors, homogenizers) or high flow rate processes (pilot plant and full production scale).
Recognizing that a central goal of process development scientists and engineers is to scale up their PAT tools from the lab to the plant, Wyatt is committed to further devel-oping RT-MALS technology to support larger scales and higher flow rates, so that PAT knowledge obtained in the process development lab can be utilized during all phases of drug product commercialization.
References
1Wen, J., Arakawa, T. and Philo, J.S. “Size-Exclusion Chromatography with On-Line Light-Scattering, Absorbance, and Refractive Index Detectors for Studying Proteins and Their Interactions”, Anal. Biochem. 240(2):155-166 (1996). https://doi.org/10.1006/abio.1996.0345
2Mendichi, R. and Schieroni, A.G. “Fractionation and characterization of ultra-high molar mass hyaluronan: 2. On-line size exclusion chromatography methods”, Polymer 43(23):6115-6121 (2002). https://doi.org/10.1016/S0032-3861(02)00586-4
3Juraszek, J. et al. “Stabilizing the Closed SARS-CoV-2 Spike Trimer”, Nature Communications 12, 244 (2021). https://doi.org/10.1038/s41467-020-20321-x
4Citkowicz, A. et al. “Characterization of virus-like particle assembly for DNA delivery using asymmetrical flow field-flow fractionation and light scattering”, Anal. Biochem. 376(2):163-172 (2008). https://doi.org/10.1016%2Fj.ab.2008.02.011
5McIntosh, N.L. et al. “Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering”, Scientific Reports 11, 3012 (2021). https://doi.org/10.1038%2Fs41598-021-82599-1
6Mildner, R. et al. “Improved multidetector asymmetrical-flow field-flow fractionation method for particle sizing and concentration measurements of lipid-based nanocarriers for RNA delivery”, Eu. J. Pharm. Biopharm. 163, 252-265 (2021). https://doi.org/10.1016%2Fj.ejpb.2021.03.004
7Patel, B.A. et al. “Multi-angle light scattering as a process analytical technology measuring real-time molecular weight for downstream process control”, mAbs 10(7):945-950 (2018). https://doi.org/10.1080/19420862.2018.1505178