AAV titer and empty-full ratio
AAV titer (Cp), also referred to as the AAV concentration, physical titer or capsid titer, is directly related to dosage and thus of critical importance to ensure safe and effective performance.
- Size-exclusion chromatography with multi-angle light scattering (SEC-MALS) offers comprehensive characterization, including quantification of both AAV capsid titer (Cp) and genome titer (Vg) along with the corresponding AAV empty/full ratio.
- Dynamic light scattering (DLS) is uniquely suited for measuring total titer, quickly and non-invasively.
The results are robust and reliable across all AAV serotypes, without the need for finicky primers, complex sample preparation, or calibration curves.
Capsid Titer (Cp), genome titer (Vg) and AAV empty/full ratio by SEC-MALS & FFF-MALS
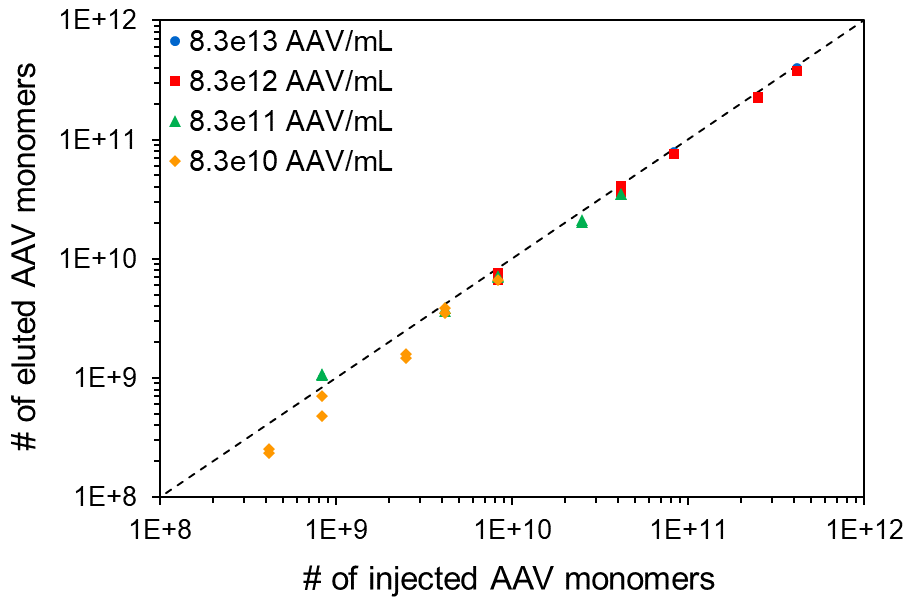
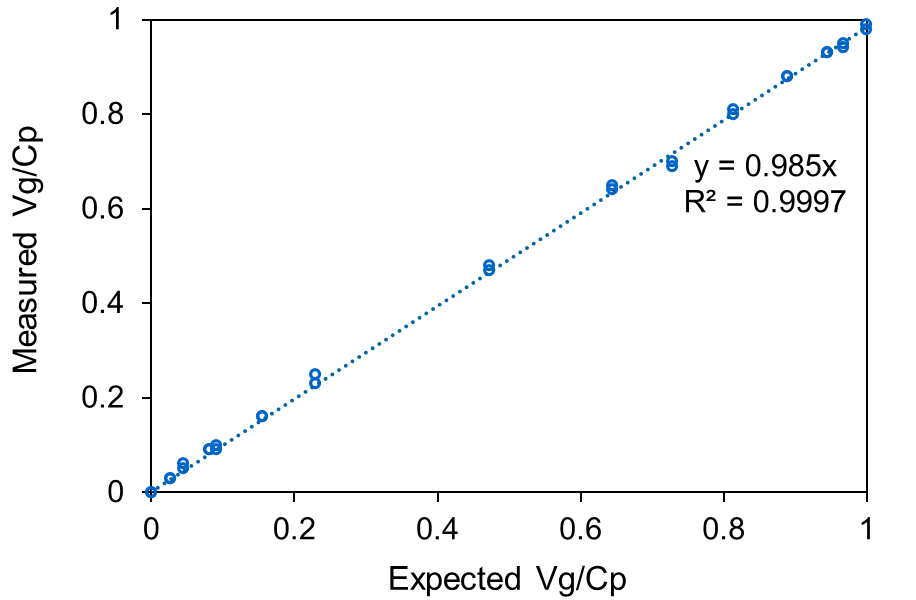
SEC-MALS analysis of AAV CQAs simultaneously provides the total AAV capsid, AAV genome concentrations, and the ratio of full-to-total (Vg/Cp), with excellent linearity, accuracy, precision, and sensitivity.
A SEC-MALS system comprising a DAWN™ MALS detector, an Optilab™ differential refractive index (dRI) detector and HPLC modules constitutes a GMP-compliant platform solution for viral vector characterization. The system readily determines the genomic and capsid titers (Vg, Cp) of an AAV sample in one short run. FFF-MALS offers similar analyses and is beneficial for quantifying AAV aggregates larger than 50 nm.
The Viral Vector Analysis procedure incorporated into the ASTRA™ software provides excellent precision in Vg/Cp, ± 3%, with a minimum quantifiable change of ± 5%. ASTRA calculates
- Total AAV concentration
- Concentration of empty capsids
- Concentration of full capsids
- Full-to-total ratio
for every slice of the chromatogram, quantifying the entire sample or specific subpopulations. The results are displayed in customizable graphs, tables, and reports that may be exported to multiple standard formats.
Capsid empty/full ratio is a critical quality attribute (CQA) of AAVs. AN1617: Quantifying quality attributes of AAV gene therapy vectors by SEC-UV-MALS-dRI describes how SEC-MALS determines empty/full ratio and other CQAs.
How does SEC-MALS compare to other common techniques for AAV empty/full ratio determination?
SEC-MALS Viral Vector Analysis results compare favorably with the theoretical % empty and with more labor and training-intensive AAV titer assays like analytical ultracentrifugation (AUC) and charge detection mass spectroscopy (CDMS). In contrast, the empty % obtained from UV absorbance methods, cryo-EM, and the Vg/Cp titer ratio from a combination of PCR and ELISA deviate from the theoretical values and the results from SEC-MALS. Compared to other standard methods, SEC-MALS has the added benefits that it is rapid, requires minimal sample handling or training, uses 21 CFR Part 11 compliant software, and is implementable in a cGMP environment.
For an in-depth discussion of how SEC-MALS compares to other standard techniques for AAV capsid content determination, please see this publication by Pfizer in collaboration with Indiana University and Gravity Diagnostics in Molecular Therapy: Methods & Clinical Development.
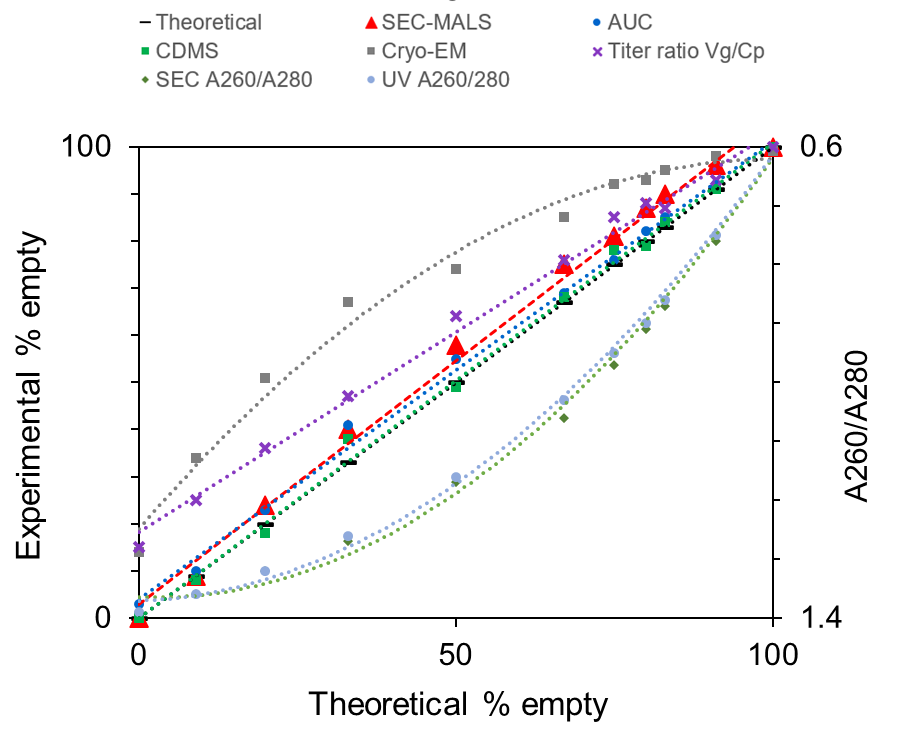
Evaluation of capsid content measured by different techniques. Adapted with permission from Werle, A.K. et al. (2021) Molecular Therapy: Methods & Clinical Developments. 23, 254-262.
Determining total AAV titer (Cp) by DLS
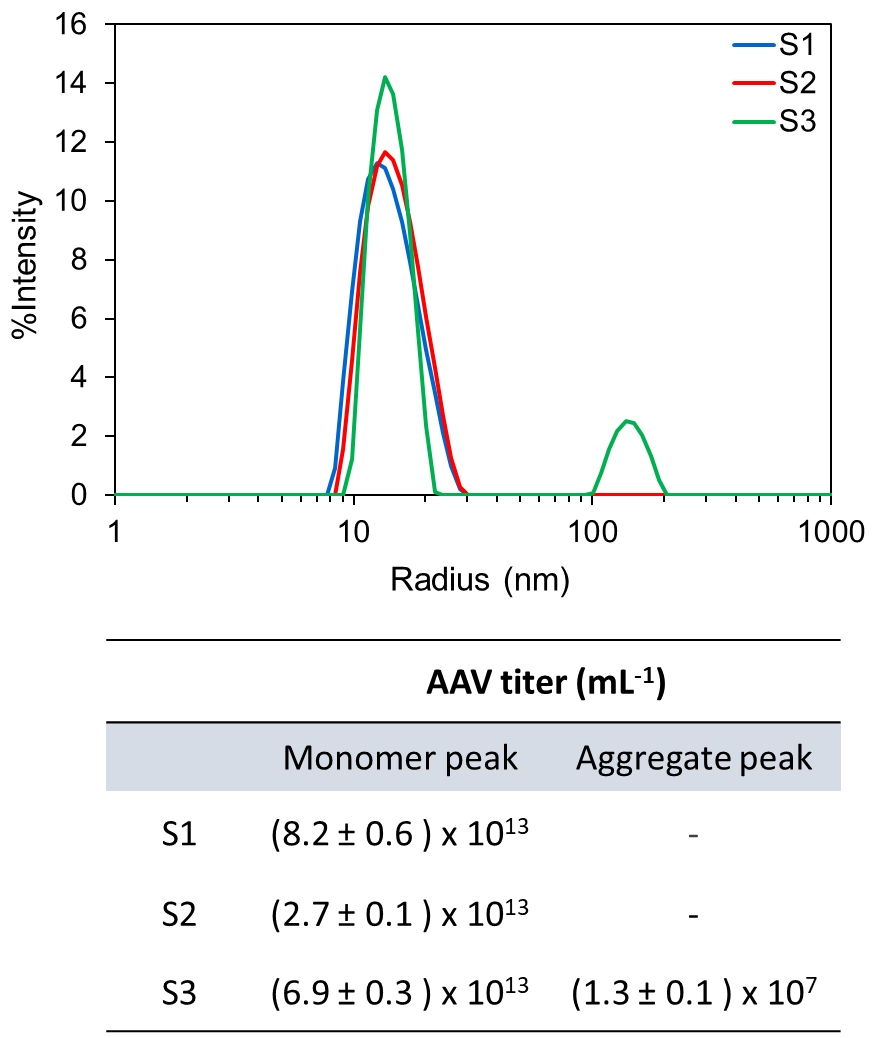
DYNAMICS determines the AAV concentrations of multimodal samples, such as sample S3, as well as monomodal samples, such as S1 and S2.
Direct measurement of AAV capsid titer can be accomplished in a few seconds using the DynaPro™ Plate Reader or DynaPro™ NanoStar™. In these instruments, simultaneous measurements of size and total scattered intensity are used to determine accurate viral concentrations. The technique is suitable for small viral vectors, like AAVs, as well as larger viruses and lipid nanoparticles. As a batch measurement, DLS enables the detection and quantitation of larger species that may be removed or disrupted under chromatography conditions.
The AAV concentration measured by DLS agrees well with other quantitative techniques, including SEC-MALS. AAV Application Notebook has several application notes that describe characterizing AAVs with light scattering.
Determining viral titer by DLS requires knowledge of the refractive indices (RI) of the viral particles and the buffer. Wyatt offers detailed technical notes to our customers providing this information for a few virus types including empty and full AAV, and other delivery vehicles such as lipid nanoparticles.



