
Analytical techniques based on light scattering are essential for quantifying quality attributes and other characteristics of gene therapy nanoparticles. Dynamic light scattering (DLS) and multi-angle light scattering (MALS) coupled to separation technologies (SEC-MALS, FFF-MALS) reveal molar mass and size, aggregation, physical titer, empty:full ratio and stability.
Continue reading to learn about Wyatt solutions to industry and regulatory needs for AAVs and other DNA/RNA delivery modalities. Light scattering techniques are simple to operate and enhance productivity while directly accessing the core biophysical properties that define these CQAs.
High-throughput size, stability, and quantity
Dynamic light scattering (DLS) provides rapid, low-volume, non-destructive measurements of a wide range of delivery vehicles, including adeno-associated virus (AAV), lentivirus, and lipid nanoparticles. The DynaPro™ Plate Reader is the premier tool for high-throughput determination of product quality, stability, and quantity, directly in microwell plates. The DynaPro™ NanoStar™ enables the same measurements in a quartz cuvette with volumes as low as 2 µL. Our white paper WP5003: Automated dynamic and static light scattering in microwell plates for fast, productive development of biologics highlights key functionality of DLS for many classes of biotherapeutics.

Viral vector quantification
Direct measurement of total virion concentration can be accomplished in a few seconds using the DynaPro Plate Reader or NanoStar. The hydrodynamic size measured by DLS combines with simultaneous intensity measurement by static light scattering (SLS) to determine accurate particle concentrations. The technique is suitable for small viral vectors, like AAV, as well as larger viruses and lipid nanoparticles.
DLS measurements of three AAV samples, S1, S2 and S3, indicate that one of them (S3) contains a small fraction of large aggregates with radius >100 nm. This aggregate population is detected and quantified, even though its concentration is a small fraction of the total number of particles. The particle concentration in the DynaPro instruments agrees well with other quantitative techniques, including SEC-MALS (more details below). Importantly, batch characterization with DLS enables detection and quantitation of larger species that may be removed or disrupted under chromatography conditions.
Details are available in the application note AN5007: Characterization of AAV-based viral vectors by DynaPro DLS/SLS instruments.
Non-viral vector quantification
Similar measurements may be carried out for non-viral vectors such as lipid nanoparticles encapsulating nucleic acids. See our page Solutions for Lipid Nanoparticle Characterization for more information.
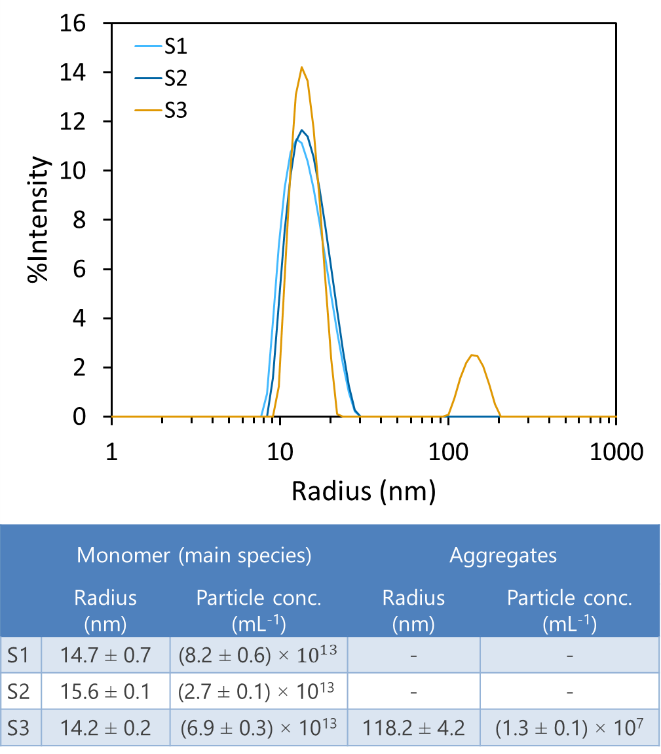
DLS analysis of three AAV samples, indicating size and concentration. S3 exhibits a population of large aggregates.
Formulation screening
The ability to screen gene therapy formulations at early stages of development ensures that only the most suitable candidates are moved through the development pipeline. Measuring the size and size distributions with DLS provides a quick assessment of the degree of aggregation and the stability of a sample.
With the suite of analyses and visualization tools provided by the DYNAMICS™ software, scientists can easily determine multiple quality attributes to develop the most stable, robust product possible. These analyses include the following:
- Screen size and size distribution of product and aggregates
- Identify SEC mobile phase or formulation buffers that promote well-assembled product
- Measure colloidal stability and propensity to aggregate
- Determine conformational stability via aggregation temperature or time to aggregate
- Conduct complete study of freeze-thaw cycles on the same plate using the same set of samples
Learn more about applying Wyatt’s DLS tools to high-throughput screening of biologics in the webinar “Quantification of viral and non-viral vector CQAs”.
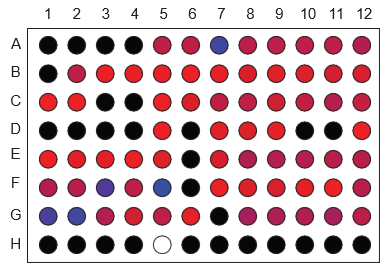
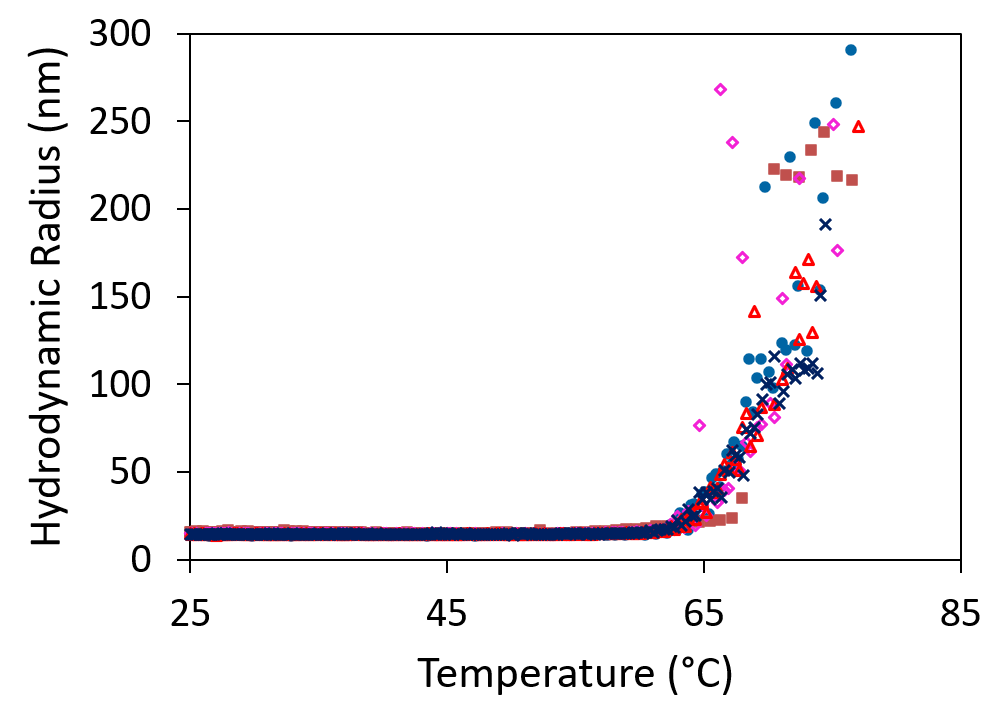
Screening of biologics formulations in a microwell plate provides rapid indication of aggregation (top) and aggregation onset temperature (bottom).
Quality and Quantity of AAV, genetic payload, and associated proteins
Size-exclusion chromatography (SEC) combined with multi-angle light scattering (MALS) is ideal for separation, characterization, and quantification of a wide range of gene therapy vectors, their genetic payload, and other proteins associated with gene therapy development. This method uses a DAWN™ MALS detector combined with an Optilab™ differential refractive index (dRI) detector and HPLC UV module to measure absolute molar mass, size, and concentration of proteins, nucleic acids, and biological nanoparticles.
A wide variety of Wyatt columns are available for complete characterization of small proteins and nucleic acids. The columns sizes range from less than a few kDa all the way up to small viruses, typically 50 nm in diameter. For more information, download our Accessories & Services Guide.

A complete SEC-MALS system includes Wyatt’s DAWN MALS detector, Optilab dRI detector and most industry-standard HPLC pumps, autosamplers and UV detector.
Molar mass and size distributions
Molar mass and size measurements with SEC-MALS are not limited to proteins. Short oligonucleotides, polysaccharides, whole plasmids, and small bionanoparticles can be fully characterized with SEC-MALS. This technique provides invaluable information about the homogeneity of your samples, degree of conjugation, and aggregation.
Here we show the measured molar mass for two plasmids, one 12 kbp and the other 6 kbp. The uniform molar mass distribution across the peak, agreeing well the expected sequence results. The increase in RMS radius on the leading edge of the peak may hint at a small number of aggregates or may suggest coelution of nicked or linear conformation with the same molar mass but larger radius.
The range of characterization is limited only by the range of the SEC column. MALS measurements for larger viruses and nanoparticles are readily achievable with FFF-MALS (see below). Learn more about how SEC-MALS in WP1615: SEC-MALS for absolute biophysical characterization.
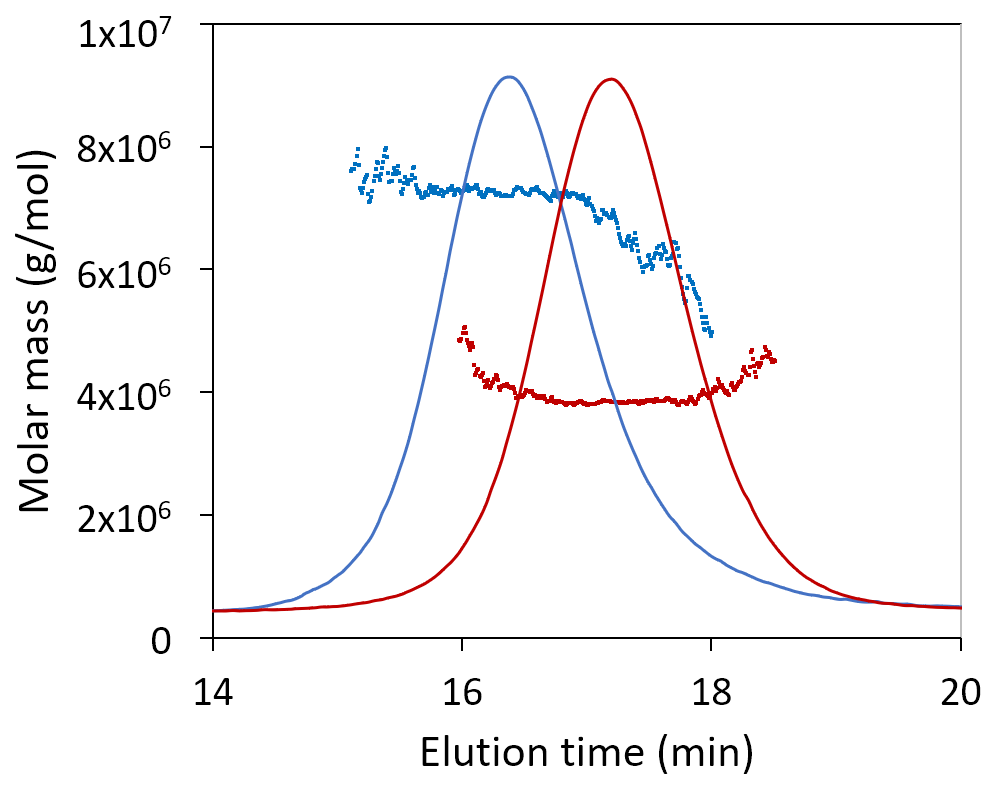
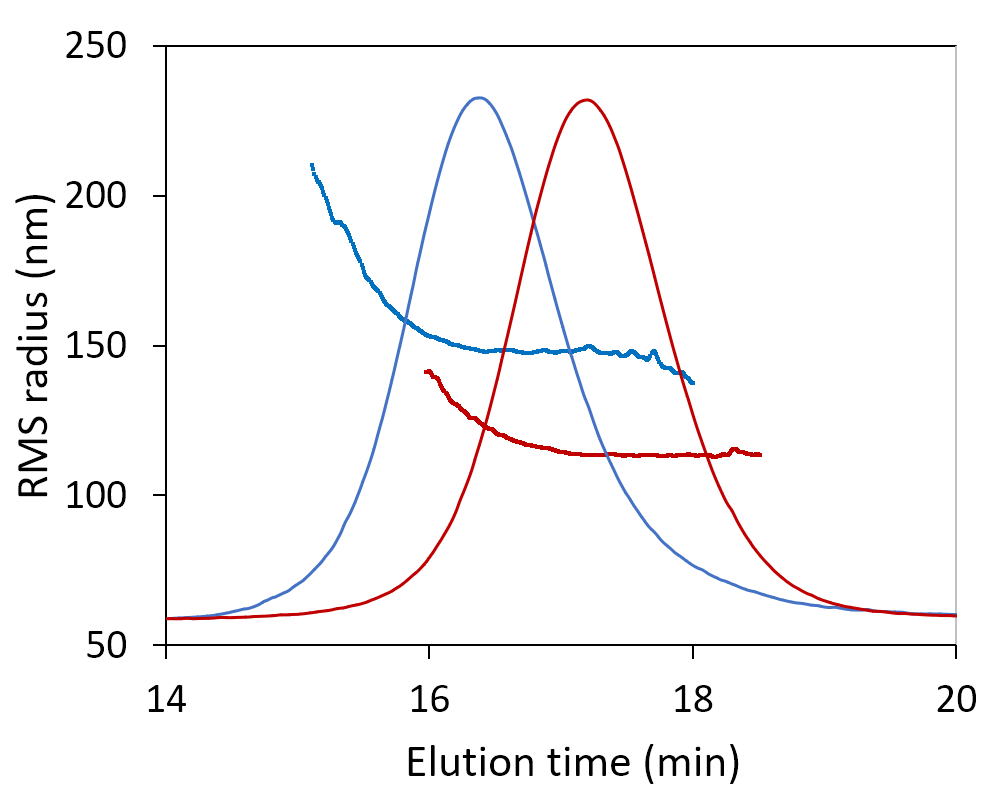
SEC-MALS analysis of 6 kbp and 12 kbp plasmids determines their molar masses and radii. Increasing size at constant molar mass (blue) may be indicative of aggregates or of monomers with elongated conformation.
AAV Critical Quality Attributes
To ensure safe and effective gene therapy using adeno-associated virus as a delivery vector, the product must meet a set of quality attributes including concentration and content.
Total particle concentration, directly related to dosage, is of critical importance. SEC-MALS is uniquely suited for measuring the total particle concentration of these small viral vectors; results are robust and reliable across all serotypes without the need for finicky primers or complex sample preparation. SEC-MALS analysis of capsid content determines the number of full and empty virions.
The Viral Vector Analysis method incorporated into ASTRA™ software provides direct measurement of total particle (capsid) concentration and capsid content. SEC-MALS results compare favorably to other techniques, and the results can be used to validate PCR or ELISA assays. In addition to the fraction of full and empty capsids, SEC-MALS determines the molar masses of the protein and nucleic acid portions. Combined with ASTRA’s 21 CFR Part 11 compliance option, SEC-MALS is readily positioned for lot-to-lot comparisons or release assays of gene therapy products.
Learn more details about these measurements in our application note AN1617: Quantifying quality attributes of AAV gene therapy vectors by SEC-UV-MALS-dRI.
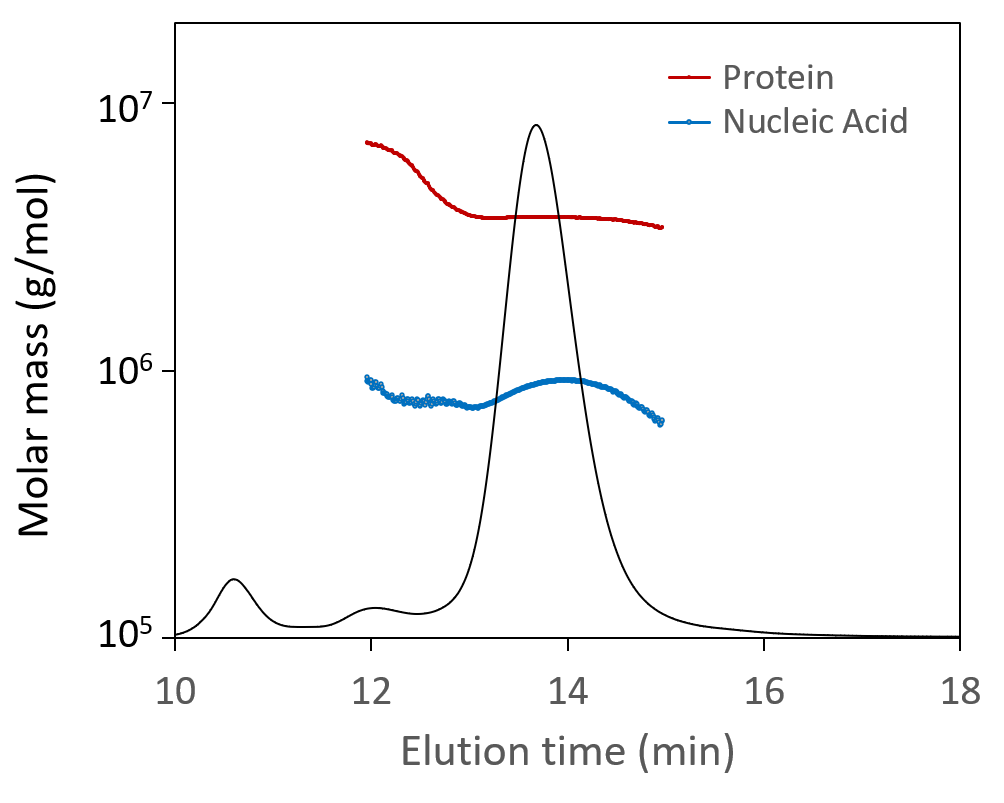
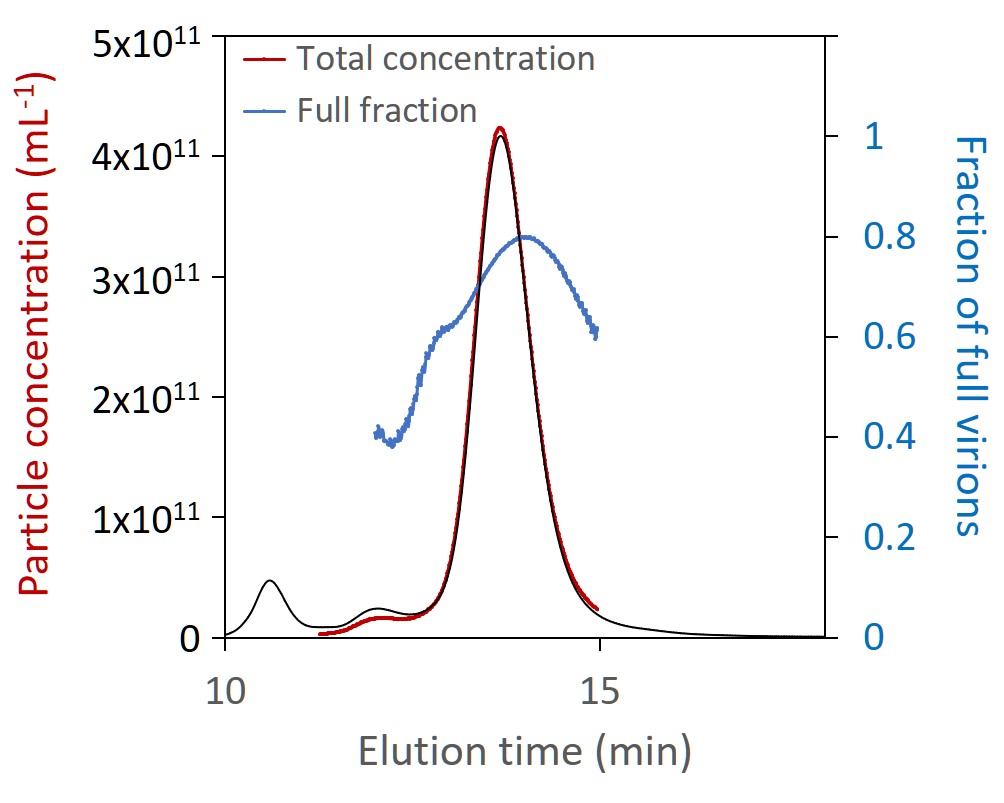
SEC-MALS analysis of AAV CQAs simultaneously provides the molar masses of capsids and nucleic acids (top) and total capsid concentration and fraction of full capsids (bottom), both overlaid on light scattering chromatograms.
Aggregates, large viruses, and mRNA
While SEC-MALS measures quality attributes across a wide range of sizes, the technique is limited with respect to larger particles and aggregates. This is because SEC columns may remove or shear these species, leading to incomplete characterization and quantitation.
Field-flow fractionation (FFF) with the Eclipse™ FFF system provides gentle separation in an open channel without a stationary phase. This removes the possibility of the sample being degraded or removed by the separation matrix. FFF coupled with MALS provides accurate size, molar mass, and particle concentration for bionanoparticles up to ~1 µm in diameter. As mentioned in the page Solutions for Lipid Nanoparticle Characterization, FFF-MALS can also characterize encapsulation efficiency and the payload of non-viral vectors.
Download our publication list to find examples of how FFF-MALS has been applied to the characterization of nano-drug-delivery vehicles.
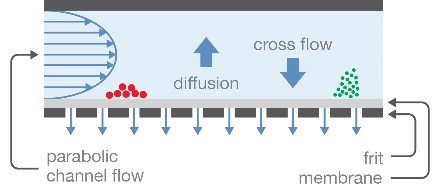
Field-flow fractionation separates particles according to hydrodynamic radius, in an open channel.
Characterization over a wide range of particle sizes
Lentivirus, lipid nanoparticles, and mRNA are often too large to elute from an SEC column. In this case, FFF-MALS is ideal for separation and characterization.
When analyzed by SEC-MALS, a polydisperse self-amplifying RNA (saRNA) appear to have a weight-average molar mass, Mw, of 2 × 106 g/mol. However, FFF-MALS reveals a much wider size distribution. In fact, the true Mw is an order of magnitude higher, and the fraction of material seen by SEC-MALS accounts for <20% of the entire sample.
ASTRA’s Number Density method provides direct measurement of particle size and concentration derived from the light scattering intensity. These calculations can be applied to a variety of common gene delivery vehicles, including viruses, virus-like particles, and lipid nanoparticles. Learn more about the sensitivity and linearity of this method in this paper published by scientists at CDC: Quantitation of influenza virus using field flow fractionation and multi-angle light scattering for quantifying influenza A particles.
Wyatt’s solutions for characterizing a variety of gene therapy products are described in the brochure Characterization of Biologics and Complex Drugs.
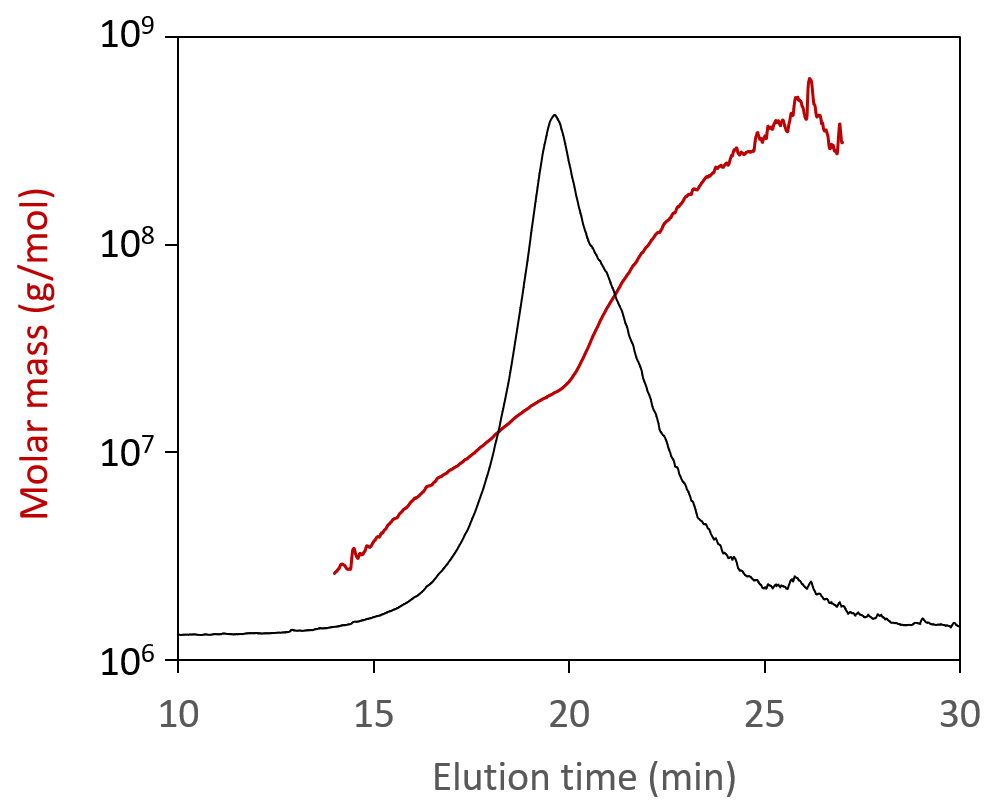
Molar mass values measured for self-amplifying RNA (saRNA), overlaid on the FFF-MALS fractogram.
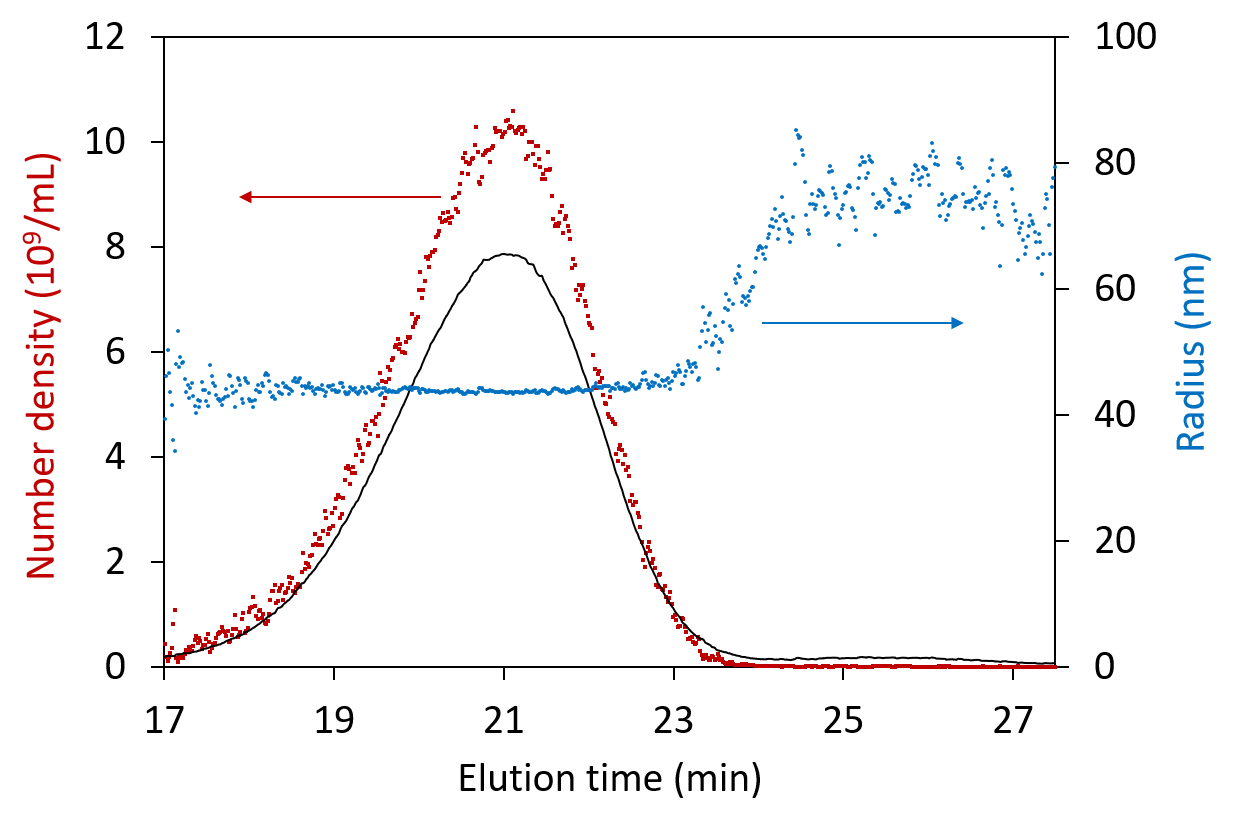
Analysis of particle concentration and radius in each eluting fraction across the peak, shown for viruses separated by FFF.
Orthogonal quantification of aggregates
Although small viruses, like AAV, are well-characterized by SEC-MALS, the possibility that large aggregates are being filtered out by the column still exists. FFF-MALS is an ideal technique for resolving and quantifying these aggregates and providing orthogonal characterization.
In this FFF-MALS fractogram, the aggregates are clearly separated from the monomer and dimer AAV and elute at ~25 minutes retention time. The size range for the peak measured by FFF-MALS spans radii of ~20 nm to ~100 nm, and the peak contained 1.89 × 1014 particles, as measured by ASTRA’s Number Density method. However, the vast majority of these were removed by SEC.
Learn more about FFF-MALS analysis of AAV aggregates in our application note AN2003: Quantifying AAV aggregation and quality attributes by FFF-MALS.
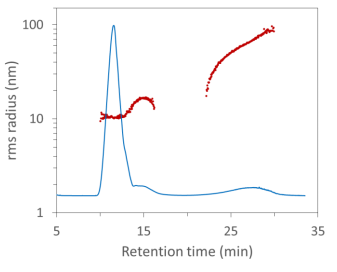
FFF-MALS analysis of AAV monomer, dimer and aggregates, differentiated by radius. The larger aggregates are filtered out by SEC and hence not quantified by SEC-MALS.
AAV Method Implementation & Training
Sensitive instrumentation combined with powerful software solutions are key for characterizing key gene therapy attributes, but they are not the whole story. You need a proven method to utilize these tools to their fullest, and that method needs to be transferrable from development throughout the product life cycle.
Wyatt Technology now offers a special AAV Method Implementation and Training service to help get you up and running with the SEC-MALS Viral Vector Analysis method. As part of this service, an application specialist will come onsite with a turnkey SEC method and standard operating procedure to ensure you are successful from the beginning.
The AAV Method Implementation & Training may be combined with an onsite PM or IQ/OQ to make sure you are up and running with minimal downtime. An invaluable resource for this method is SOP Guidance Manual: Critical Quality Attributes of AAV by SEC-MALS which serves as a comprehensive handbook for customizing a platform method, suitable for use across R&D, production. QC and comparability studies.
