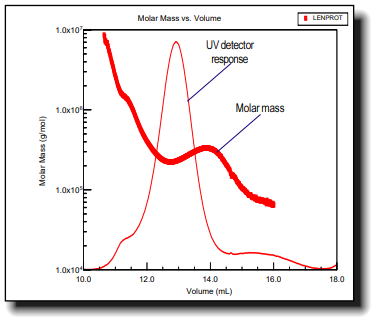
This application note reveals how you can determine conformation, and conformational changes by coupling a MALS detector (either a DAWN or miniDAWN) to the liquid chromatograph.
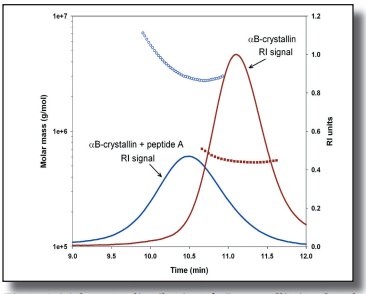
To determine the binding stoichiometry and the oligomeric state of the selected Fab fragment in complex with its target, we have used the powerful method of multi-angle light scattering in combination with size exclusion chromatography (MALS-SEC). This information is instrumental in further biophysical studies and co-crystallization experiments. Additional information such as weight fraction of protein and detergent in the protein-detergent micelle are obtained in the same experiment when using both UV and RI-detectors.
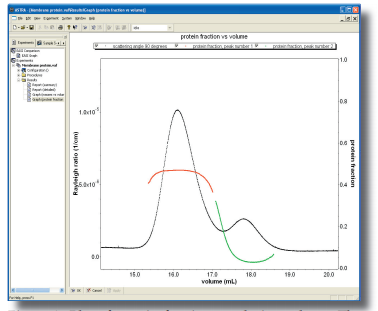
In this application note we use SEC-MALS and our ASTRA software to study the interaction of protein fragments with intact crystallins sheds light on the mechanism of protein aggregation in vivo.
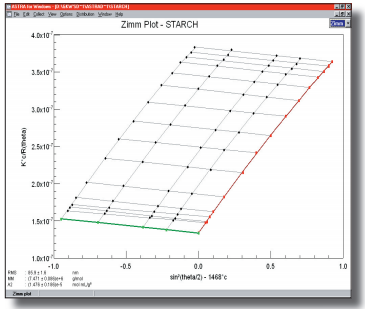
Polysaccharides (of which starch is an important example) are carbohydrates whose molecules consist simply of a number of monosaccharide residues, which are bound together. These molecules are frequently very large in molar mass and, as such, may not lend them selves to separation via size-exclusion chromatography (SEC) because they elute beyond the exclusion limits of currently-available columns. When this is the case, Wyatt Technology’s DAWN can be employed in a batch or “micro-batch” mode to produce weight-average molar masses and z-average sizes for very high molar mass starches, with out separation.
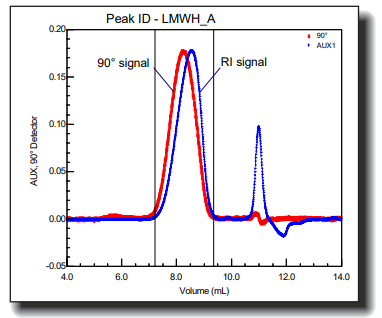
Heparin is well-known as an anti-coagulant, anti-thrombotic drug. Chemically, it is a linear polysaccharide that is derived from animal tissues. For some time it has been known that heparin is not a homogeneous substance; rather, it is a heterogeneous mixture of molecules ranging in molar mass from less than 5,000 to more than 30,000 Daltons. Heparin can be chemically or enzymatically depolymerized to obtain low molecular weight (LMW) heparin products, which exhibit an improved pharmacological profile.
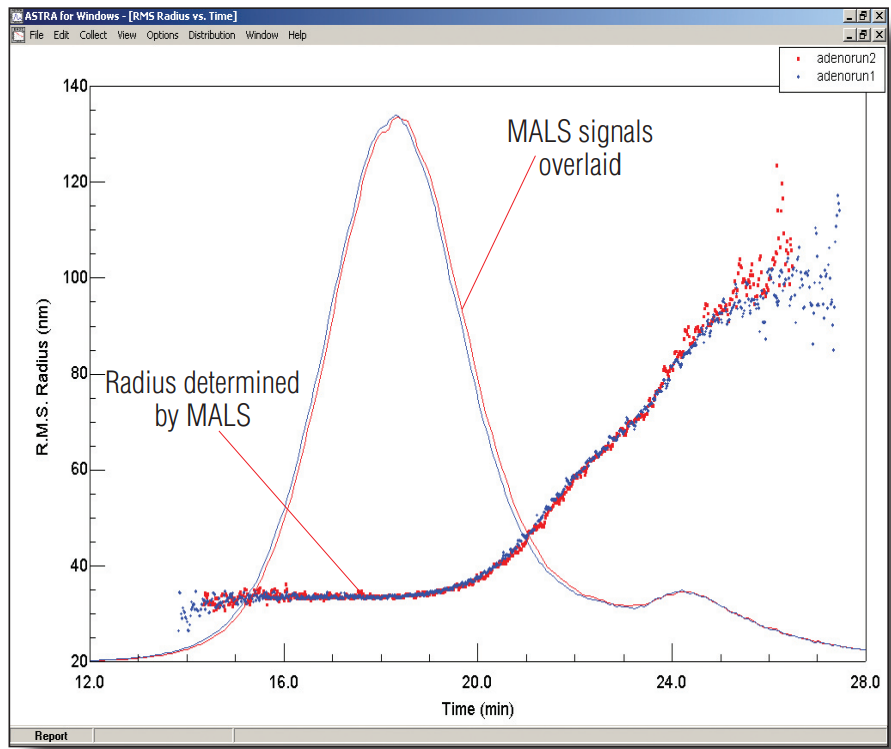
In this application note we report the results, in collaboration with the scientists at Biogen, of characterizing adenovirus particles using the Eclipse field flow fractionator (FFF) equipped with the DAWN multi-angle light scattering (MALS) detector.
This document lists key publications using AF4-MALS-DLS for the characterization of nanoparticle drug delivery systems (nanoDDS), including nanoparticles for delivering small molecules, proteins, DNA, RNA and other therapeutics.
Composition-gradient multi-angle light scattering (CG-MALS) provides direct measurement of affinity and absolute stoichiometry for a wide variety of fusion-protein complex interactions, including multivalent interactions, without the need for surface immobilization or tagging.
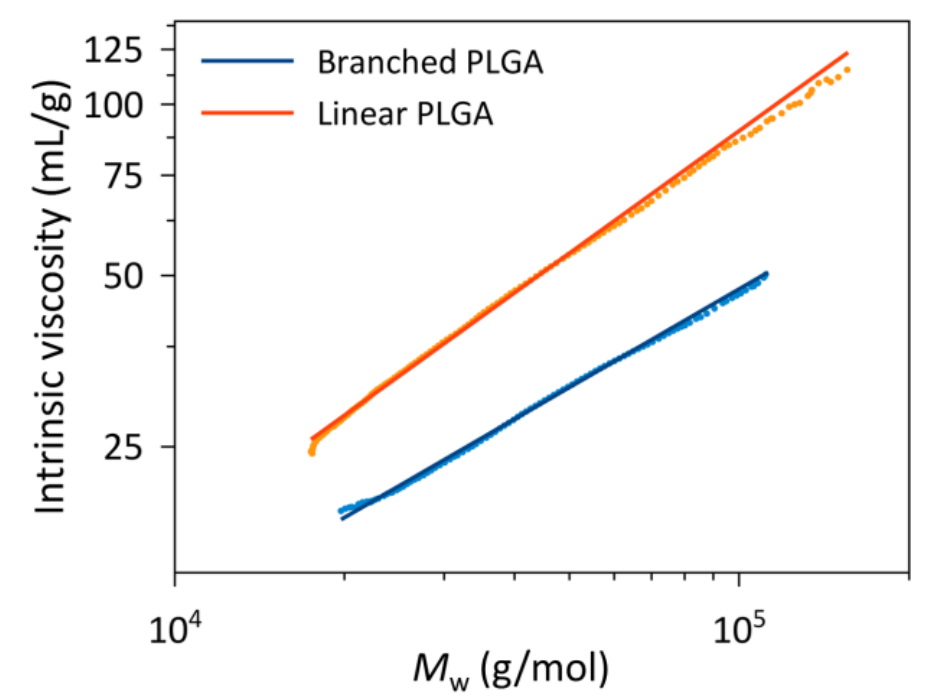
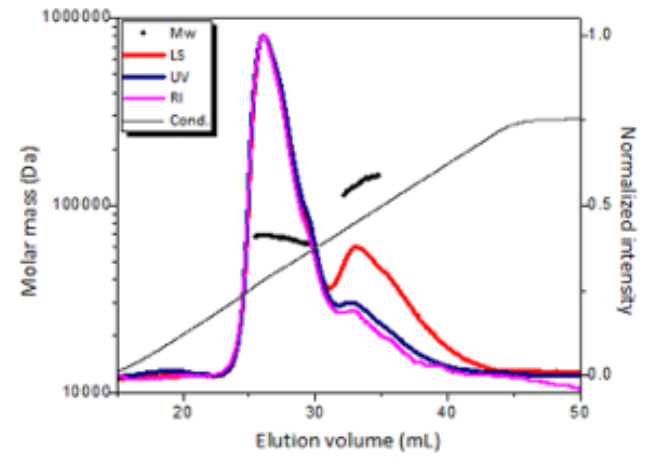
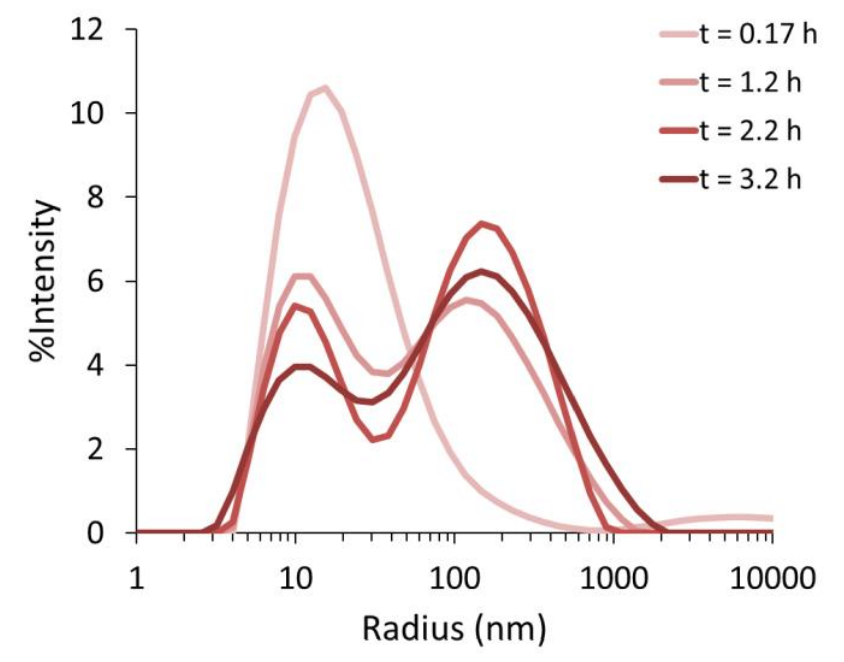
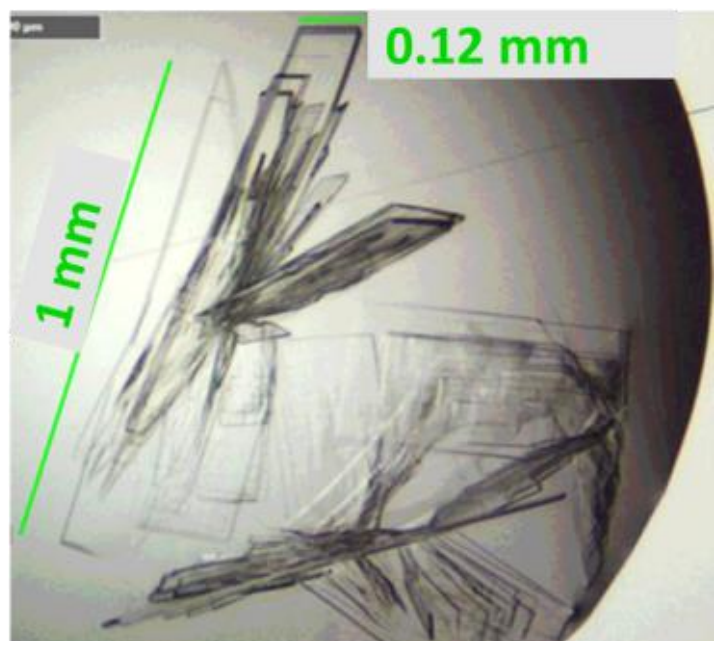
Determining the properties of PLGA is crucial in product formulation. Read more about how we used SEC-MALS with APC to calculate size and conformation along with absolute molecular weight.
Coupling of MALS to ion-exchange chromatography is useful when protein species-co-elute on SEC. This protocol shows how to perform IEX-MALS analysis of BSA.
In this work, batch dynamic and static light scattering in the DynaPro Plate Reader are used to investigate the aggregation of tau proteins, which have been observed to form neurofibrillary tangles in patients suffering from neurodegenerative diseases.
Ion-exchange chromatography is a common intermediate step in a protein purification protocol. Adding a DAWN or miniDAWN multi-angle light scattering (MALS) detector in-line with FPLC and preparative ion-exchange chromatography (pIEX-MALS) allows absolute molar mass determination of the eluting fractions in the course of the run.