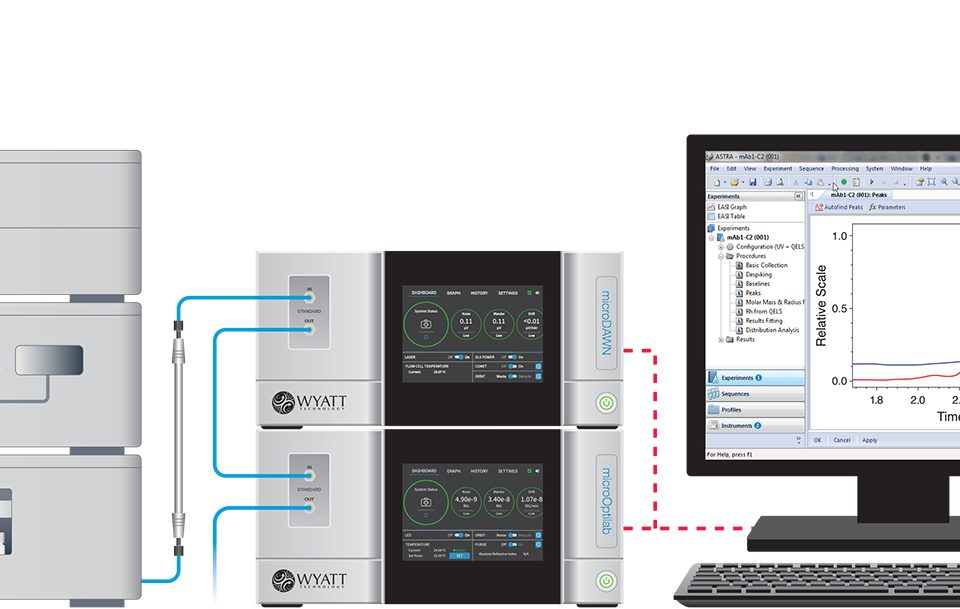
One of the primary challenges in developing effective formulations for the nanoscale delivery of therapeutics is particle characterization. Learn how to characterize nano-pharmaceuticals by reading our white paper.
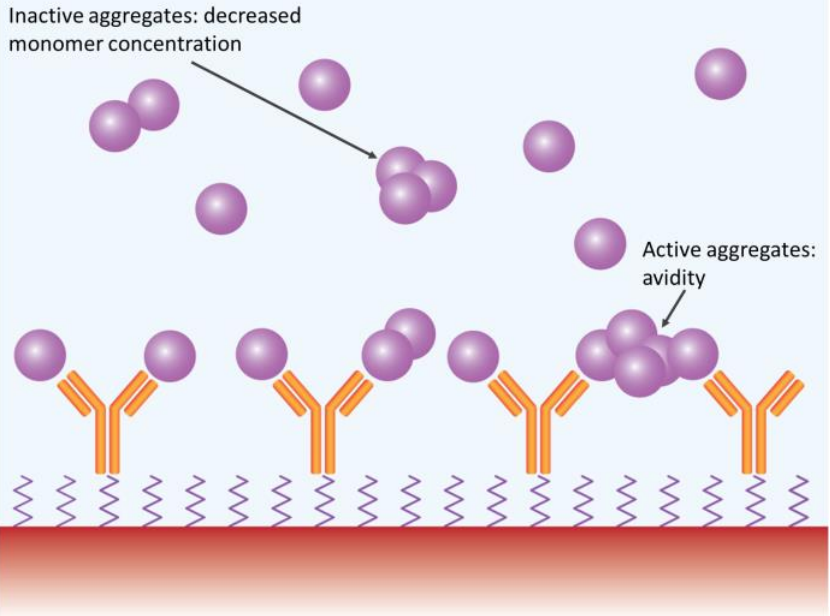
Stability is a key quality attribute in formulation studies of potential therapeutic biomolecules. In order to minimize time, effort and funds spent on stability studies, researchers rely on high-throughput screening methods that can reliably test hundreds of combinations of candidates, excipients and buffer conditions. Learn more by reading our white paper.
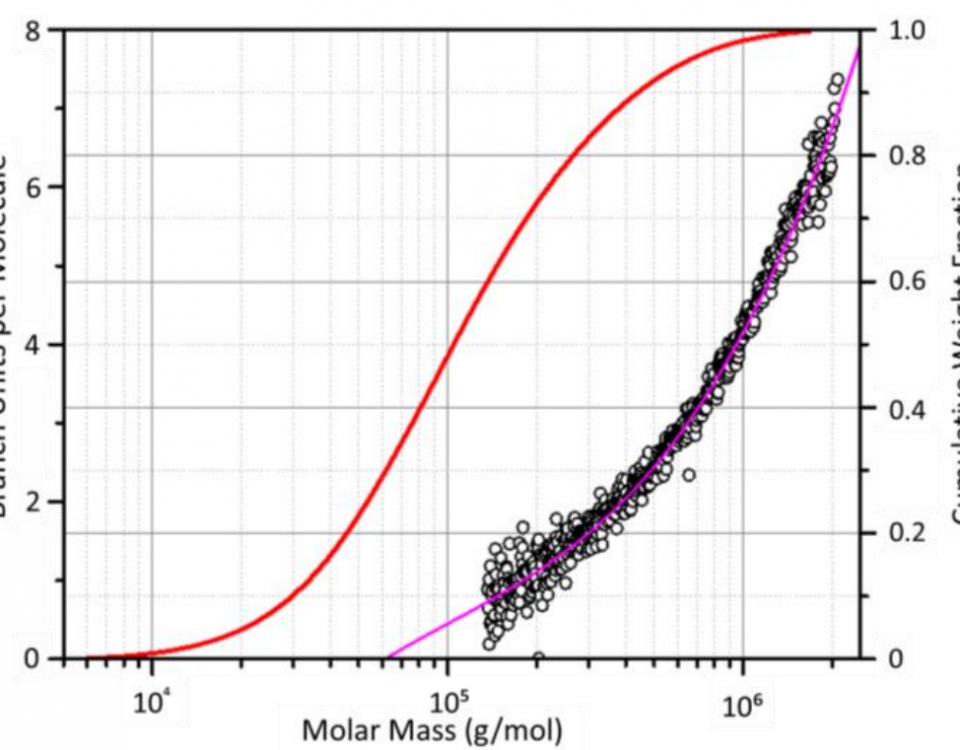
The comparison of SEC-MALS and A4F-MALS proves superior separation of large and highly branched macromolecules by A4F compared to SEC. The addition of MALS capabilities is invaluable for analyzing branched polymers. Read our white paper here.
This protocol shows how to perform basic SEC-MALS analysis of BSA to determine absolute molar mass and characterize small aggregates.
In this study, a mixture of gold nanoparticles was characterized via Hollow-Fiber Flow Field-Flow Fractionation (HF5) coupled to single-particle-inductively coupled plasma mass spectrometry (sp-ICP-MS). Read more here.
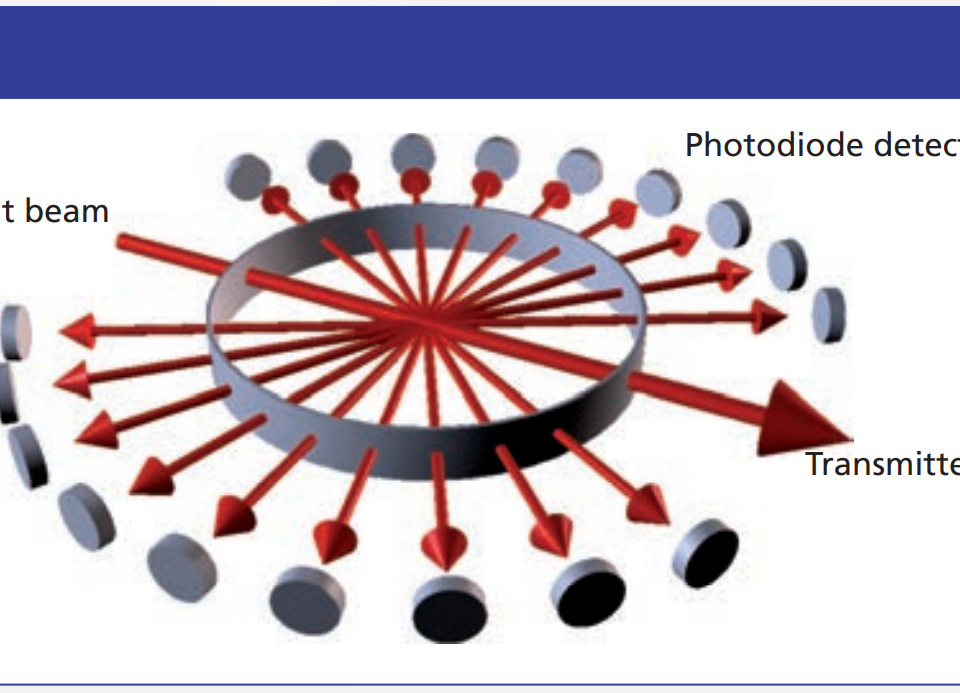
This E-book explains how light scattering works and assesses its capabilities for biotechnology applications. Read it here.
This application note demonstrates how highthroughput dynamic light scattering (DLS) with automated well-plate sampling using the DynaPro Plate Reader and DYNAMICS software enables the rapid and reliable characterization of two ADCs based on monoclonal antibodies (mAb) IgG1 and IgG2. Read it here.
Dynamic light scattering is commonly used to evaluate protein aggregation, degradation and solution quality. Learn more by reading our application note.
µSEC-MALS is necessary for UHP-SEC characterization of branched polymers, rod-like polymers and co-polymers, all of which have no appropriate column calibration standards. Read our entire white paper here.
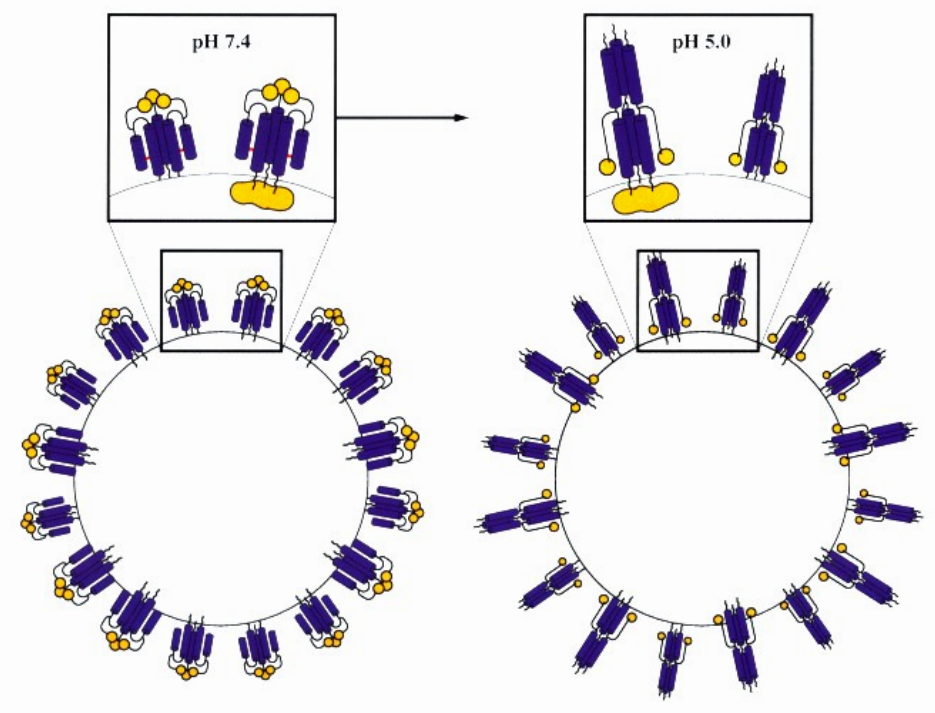
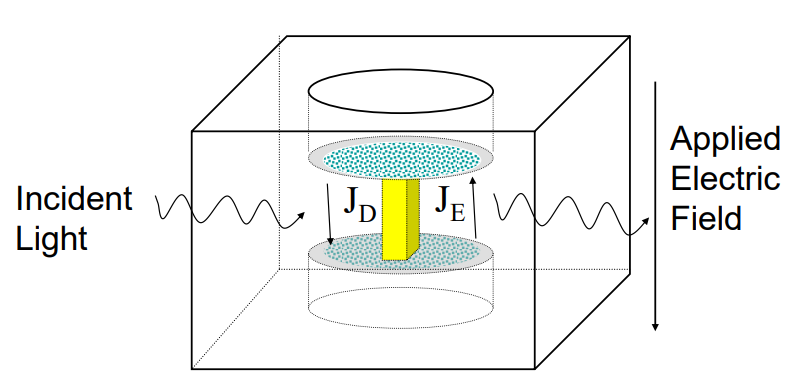
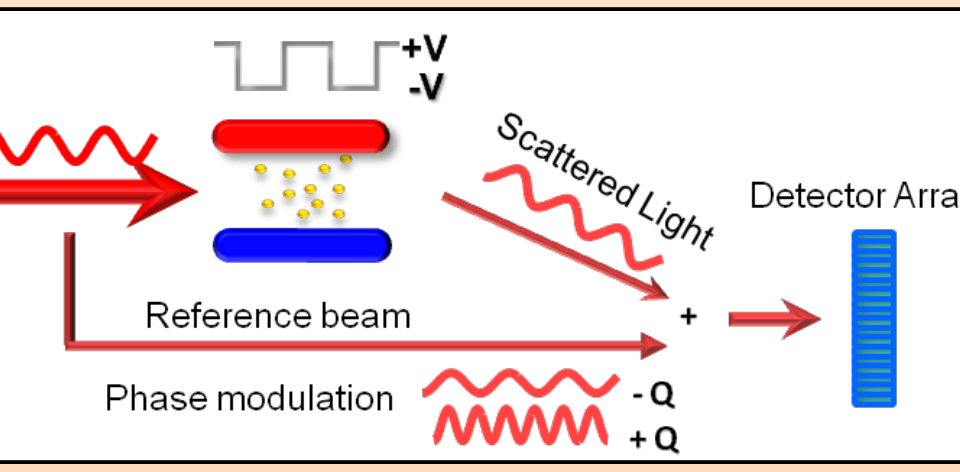
In this scientific poster we have examined the electrophoretic mobility μ, zeta-potential and effective charge of a monoclonal antibody, mAb C, and made a comparative study of protein solution analytical methods.