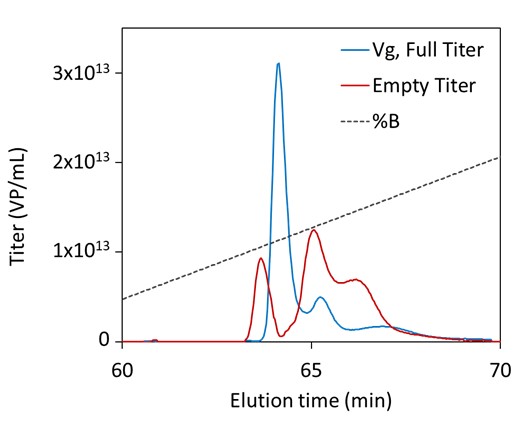
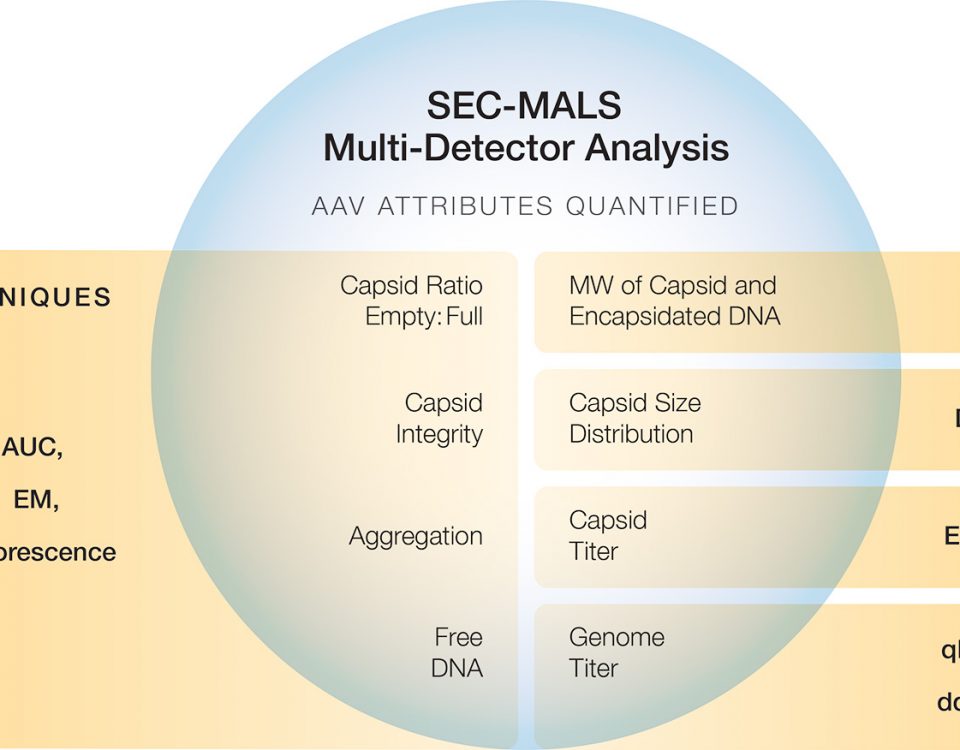
This application note demonstrates the benefits of XBridge Premier Protein and XBridge Premier GTx BEH SEC columns for multi-attribute quantitation of therapeutic antibodies, biosimilars and adeno-associated viruses (AAVs) by SEC-MALS.
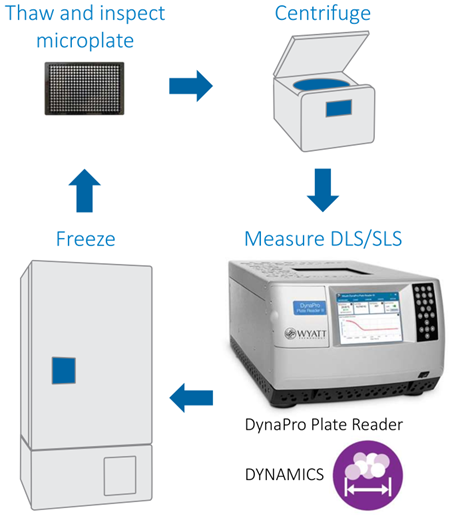
An HPLC system equipped with an autosampler enables unattended, automated measurements of electrophoretic mobility µE and hydrodynamic radius Rh with the Mobius™ instrument for dynamic and electrophoretic light scattering. A pre-programmed sequence controls the timing of injections, while the auto-inject signal triggers an Event Schedule in Wyatt’s DYNAMICS® software to collect data automatically.
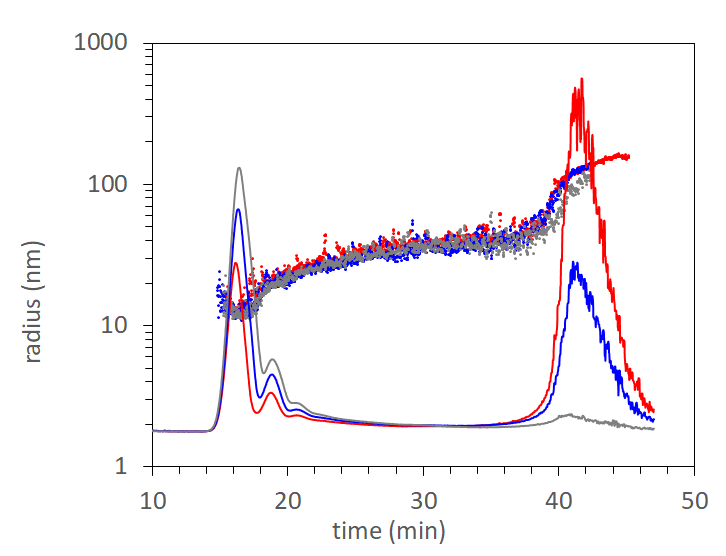
This application note compares analytical separations by size exclusion chromatography (SEC) and field-flow fractionation (FFF), along with detection by UV, fluorescence, and multi-angle light scattering (MALS). The results demonstrate that FFF-MALS is the most appropriate method for quantifying all aggregates of AAV-mediated products, from dimer, trimer, and small oligomers to large aggregates.
Freeze-thaw stability studies are a requirement for the successful development, distribution, and storage of a wide range of biopharmaceutical products.
Learn how to optimize the analysis of your viral vectors by reading our E-book.
Biopharmaceuticals, comprising monoclonal antibodies, antibody-drug conjugates, recombinant or fusion proteins, and bispecifics, among others, have recently gained commercial success and are rapidly changing the therapeutic landscape. Due to their large size and complex structures, characterizing these biotherapeutics requires robust and demanding analytical techniques. Often, traditional methods used to characterize proteins fail to deliver on either the sensitivity required or the speed desired to accelerate the drug development pipeline.
This note demonstrates the use of batch dynamic light scattering (DLS) and online multi-angle light scattering and dynamic light scattering in combination with field-flow fractionation (FFF-MALS-DLS) for characterizing large viral vectors such as lentivirus and gammaretrovirus. DLS in batch mode rapidly screens particle size distribution, concentration/titer and stability.